References have been made in the literature to the funding of clinical trials by the pharmaceutical industry. Other types of funding, however, have been less well studied.
ObjectiveTo describe the sources of funding for research by Spanish dermatology departments published in 2008.
Material and methodsA bibliometric study was performed of the research articles published by Spanish, French, and British dermatology departments and by Spanish rheumatology departments in 2008 according to MEDLINE records.
ResultsArticles published by Spanish dermatology departments received funding in 36.4% of cases. This percentage is lower than that found for the other groups studied and remained low for all different types of funding. Statistically significant relationships were found between a higher percentage of funding and a higher level of evidence, as well as between a higher level of funding by the pharmaceutical industry and the publication of research into quality of life and pharmacological treatment. Inadequate declaration of funding was observed in 57.1% of articles from Spanish dermatology departments and the role of the sponsor was not declared in any article. Similar findings were obtained for the other groups studied.
ConclusionsThe proportion of research articles published by Spanish dermatology departments that receive external funding is low, and this is associated with a lower level of scientific evidence. In order to obtain more external funding, we must improve our competitiveness.
Existen referencias en la literatura acerca de la financiación de los ensayos clínicos por la industria farmacéutica. Otros tipos de financiación han sido menos evaluados.
ObjetivosDescribir la presencia de financiación y su tipo en la investigación realizada por los Servicios de Dermatología españoles en el año 2008.
Material y métodosEstudio bibliométrico de los artículos de investigación publicados por Servicios de Dermatología españoles, franceses y británicos y Servicios de Reumatología españoles en el año 2008, indexados en Medline.
ResultadosEl porcentaje de financiación de los artículos de investigación publicados por Servicios de Dermatología españoles fue del 36,4%, siendo este porcentaje menor en comparación con los restantes grupos estudiados, y manteniéndose bajo para los distintos tipos de financiación. Existen relaciones significativas entre un mayor porcentaje de financiación y un mayor nivel de evidencia, así como entre un mayor porcentaje de financiación por la industria y los temas de calidad de vida y de tratamiento farmacológico. En un 57,1% de los artículos de investigación dermatológica española no se declara la financiación de modo adecuado y en ninguno se indicó el papel del financiador. Estos últimos hallazgos fueron similares para los restantes grupos estudiados.
ConclusiónEl porcentaje de financiación externa en los artículos de investigación publicados por Servicios de Dermatología españoles es bajo, y se relaciona con menor nivel de evidencia científica que los restantes grupos estudiados. Se propone la necesidad de aumentar nuestra competitividad para obtener mayor financiación externa.
The literature contains reports of the different types of funding used to support medical research, but most have focused on the relationship between clinical research and the pharmaceutical industry and the possible implications for quality of the study design and the results obtained.1,2 Studies of other types of funding (such as that provided by public institutions and foundations or similar entities) and other types of research (eg, basic research or clinical research other than clinical trials) are less common.
The scarcity of funds available for clinical research in Spain, together with the low production of epidemiological studies, has been previously described.3
The main aim of the current study was to assess the prevalence and type of funding of research conducted by Spanish dermatology departments. To our knowledge, this is the first study to address this question. Other aims were to assess if there were differences between the funding of clinical and basic research, to investigate the relationship between funding and level of scientific evidence or area of research, and to determine whether researchers complied with guidelines on financial disclosure. For comparative purposes and to place our findings within a context, we also analyzed publications by other groups.
Material and MethodsWe performed a bibliometric study of research articles published by Spanish, French, and British dermatology departments and Spanish rheumatology departments in 2008. The year 2008 was chosen because it was considered sufficiently recent to reflect the current situation yet sufficiently distant to ensure that all relevant articles would be adequately indexed on Medline.
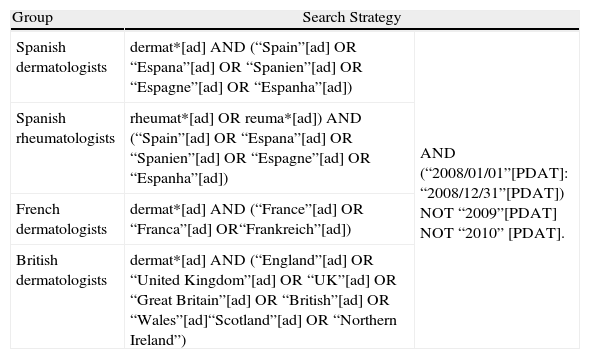
Search StrategiesWe searched the PubMed MEDLINE database in April 2010 using the methods reported by Arangeui et al.4 The search strategy used for the different groups was similar (Table 1).
Search Strategy Used to Retrieve Research Articles Published by the Different Groups Analyzed in 2008 (PubMed MEDLINE database). Search Conducted in April 2010.
| Group | Search Strategy | |
| Spanish dermatologists | dermat*[ad] AND (“Spain”[ad] OR “Espana”[ad] OR “Spanien”[ad] OR “Espagne”[ad] OR “Espanha”[ad]) | AND (“2008/01/01”[PDAT]: “2008/12/31”[PDAT]) NOT “2009”[PDAT] NOT “2010” [PDAT]. |
| Spanish rheumatologists | rheumat*[ad] OR reuma*[ad]) AND (“Spain”[ad] OR “Espana”[ad] OR “Spanien”[ad] OR “Espagne”[ad] OR “Espanha”[ad]) | |
| French dermatologists | dermat*[ad] AND (“France”[ad] OR “Franca”[ad] OR“Frankreich”[ad]) | |
| British dermatologists | dermat*[ad] AND (“England”[ad] OR “United Kingdom”[ad] OR “UK”[ad] OR “Great Britain”[ad] OR “British”[ad] OR “Wales”[ad]“Scotland”[ad] OR “Northern Ireland”) | |
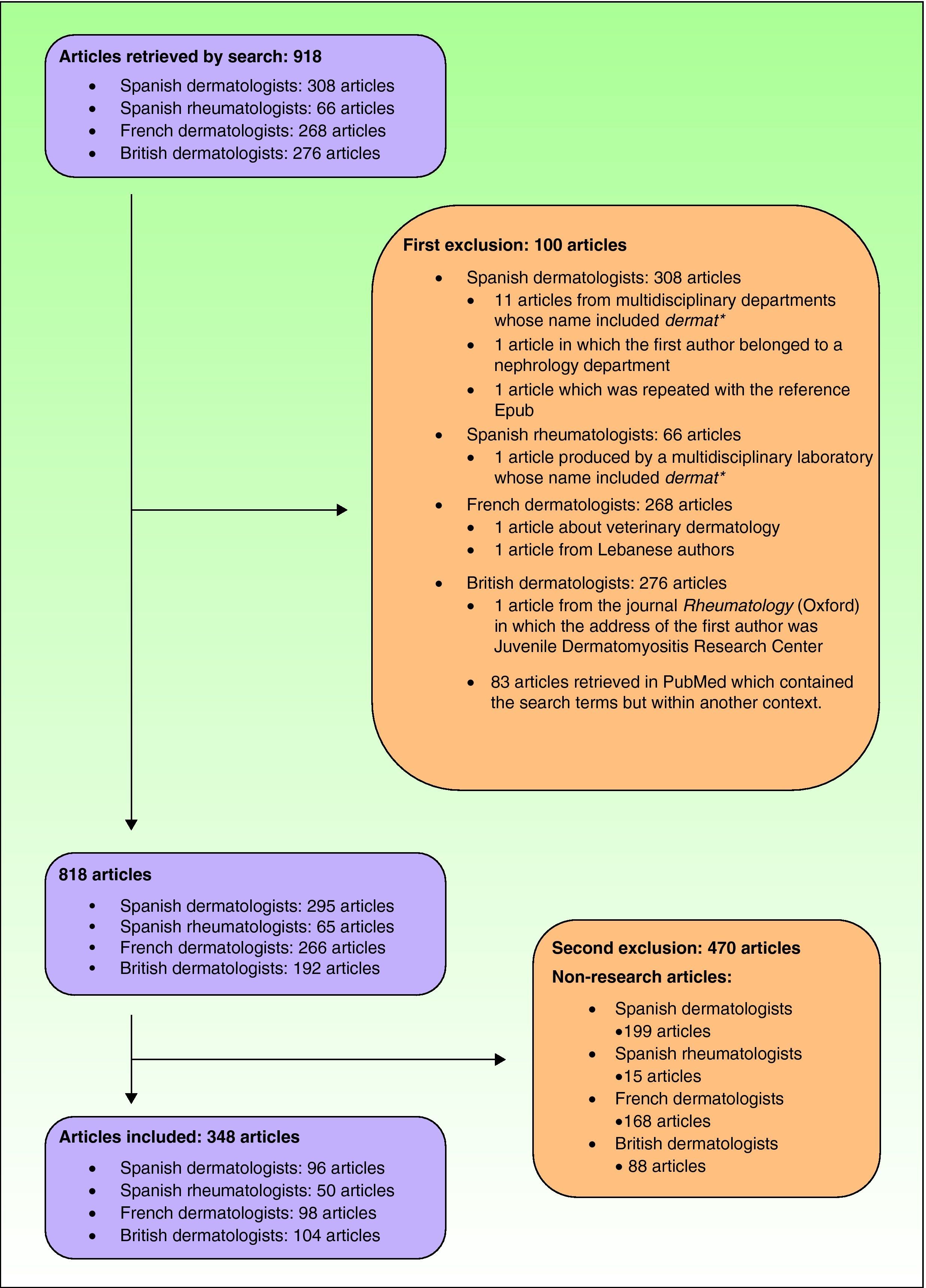
Using the filter terms Dermat*, Reumat*, and Rheumat* (as appropriate) in the first author affiliation field, we searched for research articles by Spanish, British, and French dermatology departments and Spanish rheumatology departments that were indexed in Medline in 2008 or published online in the same year in the case of electronic-only journals.
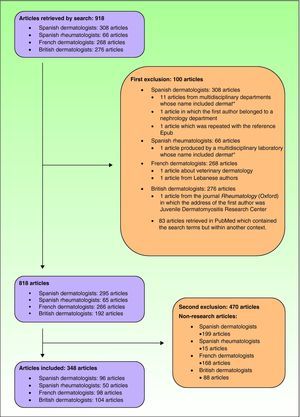
Exclusion criteriaArticles which clearly did not deal with dermatology or rheumatology topics were excluded. This exclusion was justified because the filters used also yield publications by large, multidisciplinary departments containing the terms Dermat*, Reumat*, or Rheumat*.4 Also excluded were articles that were classified as non-research articles.
Processing of DocumentsThe documents retrieved were initially grouped into 3 categories:
- 1.
Clinical research articles
- 2.
Basic research articles
- 3.
Non-research articles
Clinical research articles were further classified by level of scientific evidence using a reproducible and previously validated definition of clinical research.4Table 2 shows the definitions used to distinguish between clinical and basic research articles. Articles that did not correspond to either of these categories were classified as non-research articles.
Definitions of Clinical Research and Basic Research.
| Clinical Research | Basic Research |
| An article was considered to be a clinical research article if it was the result of a planned, organized effort and fulfilled the 3 criteria described belowa: | An article was considered to be a basic research article if it was the result of a planned, organized effort and fulfilled at least 1 of the criteria described below: |
| a) It involved patients, healthy individuals, or health care systems, or analyzed articles dealing with patients. This included studies of samples obtained from patients or healthy individuals (eg, biopsy samples, dermatoscopic images, laboratory tests). | a) It did not deal with patients, healthy individuals, health care systems, articles dealing with patients, or samples obtained from patients or healthy individuals (eg, biopsy samples, dermatoscopic images, laboratory tests, etc). It did include studies performed on nonhuman organisms. |
| b) It answered a question relevant to clinical practice, with the aim of resolving practical problems regarding patient management, including research on prevalence, etiology, diagnosis, prognosis, prevention, disease treatment, economic issues, and health care systems. It also included systematic reviews in these areas. | b) It answered a question that was not relevant to clinical practice in terms of resolving problems affecting patients. It involved research into biology, biochemistry, physiology, or pathogenesis, as well as systematic reviews in these areas. |
| c) It had a level of evidence of 4 or less based on the Oxford Centre for Evidence-Based Medicine classification system. Articles with a score of 3 or less were considered to have a high level of scientific evidence. | c) The results of the study could not be applied with guarantees of safety or efficacy to patients or healthy individuals as the consequences of the study outcomes (in terms of safety and efficacy) had not been explored in practice. |
The observers were not blinded to the names of the authors as it has been indicated that this does not produce bias in the analysis of manuscripts.5 The research articles were identified using the PubMed identifier. The following details were recorded for each article: a) research group (specialty/country); b) formulation of a question relevant to clinical practice; c) focus on patients, healthy individuals, samples, or health care systems; d) level of scientific evidence (according to the Oxford Centre for Evidence-Based Medicine (CEBM)6; e) area of clinical research, where relevant (prevalence, etiology, diagnosis, prevention, pharmacological and nonpharmacological treatments, quality of life, economics, and health care systems); f) financial disclosures; g) presence and type of funding; and h) details of the role of the sponsor (ie, level of control over study data and publication).
According to the CEBM classification system used, articles with a score of 3 or less were considered to have a high level of scientific evidence. A score of 4 was assigned to epidemiological studies and case series, and a score of 5 was assigned to case reports, commentaries, nonsystematic reviews, etc. Because these articles are more prone to random errors and errors due to bias and confounding factors, they were classified as non-research articles.
Classification of ArticlesWe analyzed the presence and type of funding by studying different sections of the articles:
- 1.
Affiliations. It was considered that funding existed if at least 1 of the authors worked at a public institution, a foundation-type institution, or a pharmaceutical company. We considered that the production of all articles involves a cost (staining of a biopsy sample, use of disposable surgical material etc.) that would be identical for public and private hospitals and for university departments. What we were interested in assessing, however, was the presence of additional, or external, funding, ie, funding other than that used to cover costs associated with routine practice. Accordingly, external funding was not considered to exist if all the authors worked at a public or private hospital or university.
- 2.
Financial disclosure section. This was considered to be any part of the article that included the term funded by (or similar). When specific mention of funding was made, a record was made of the type of funding organization.
- 3.
Conflicts of interest. Funding was considered to exist if there was mention of a relationship with a public institution, a foundation-type institution, or industry, except when it was explicitly stated that this relationship had had no direct bearing on the study.
- 4.
Acknowledgments. Funding was considered to exist if support from a public institution, a foundation-type institution, or industry was acknowledged.
- 5.
Material and methods. Funding was considered to exist if the material and methods section mentioned that the study material, or any of the material used to conduct the study, had been donated by a public or foundation-type institution or by industry.
Financial disclosure was regarded as adequate when there was an explicit financial disclosure statement in addition to a statement regarding the role played by the sponsor.7,8
The different types of funding were recorded on a Microsoft Excel spreadsheet together with links to the websites of the sponsors or to pages containing information about them. All sponsors were analyzed independently by 2 observers blinded to the corresponding articles. A third person intervened in the few cases in which were there discrepancies regarding classification. Three categories of funding were used:
- a)
Public funding: grants or funding received from government institutions such as national health institutes or local, regional, national, or international bodies, or affiliation with these institutions.
- b)
Foundation-type funding: grants or funding received from independent not-for-profit bodies such as foundations, institutes, scientific societies, patient associations, or similar, or affiliation with these bodies.
- c)
Industry funding: funding received from pharmaceutical companies or from manufacturers of the material studied.
The general study data were recorded on Microsoft Excel spreadsheets and analyzed using Stata 10 (StatCorp LP, 2009). The tests used were χ2, the Fisher exact test, and logistic regression.
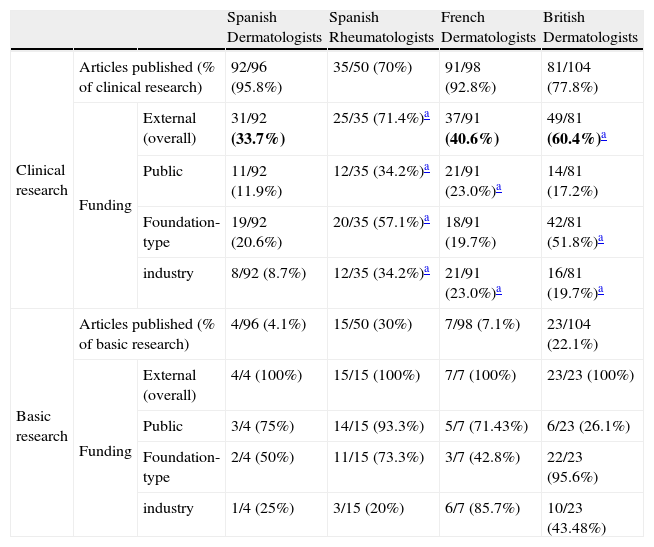
ResultsArticles Included in the StudyThe search strategy retrieved 918 articles. After applying the inclusion and exclusion criteria, the final number of articles was 348, of which 96 were publications by Spanish dermatology departments (Fig. 1). The breakdown of clinical and basic research articles published by each group is shown in Table 3.
Type of Research and Funding in Articles Published by the Different Groups Analyzed.
| Spanish Dermatologists | Spanish Rheumatologists | French Dermatologists | British Dermatologists | |||
| Clinical research | Articles published (% of clinical research) | 92/96 (95.8%) | 35/50 (70%) | 91/98 (92.8%) | 81/104 (77.8%) | |
| Funding | External (overall) | 31/92 (33.7%) | 25/35 (71.4%)a | 37/91 (40.6%) | 49/81 (60.4%)a | |
| Public | 11/92 (11.9%) | 12/35 (34.2%)a | 21/91 (23.0%)a | 14/81 (17.2%) | ||
| Foundation-type | 19/92 (20.6%) | 20/35 (57.1%)a | 18/91 (19.7%) | 42/81 (51.8%)a | ||
| industry | 8/92 (8.7%) | 12/35 (34.2%)a | 21/91 (23.0%)a | 16/81 (19.7%)a | ||
| Basic research | Articles published (% of basic research) | 4/96 (4.1%) | 15/50 (30%) | 7/98 (7.1%) | 23/104 (22.1%) | |
| Funding | External (overall) | 4/4 (100%) | 15/15 (100%) | 7/7 (100%) | 23/23 (100%) | |
| Public | 3/4 (75%) | 14/15 (93.3%) | 5/7 (71.43%) | 6/23 (26.1%) | ||
| Foundation-type | 2/4 (50%) | 11/15 (73.3%) | 3/7 (42.8%) | 22/23 (95.6%) | ||
| industry | 1/4 (25%) | 3/15 (20%) | 6/7 (85.7%) | 10/23 (43.48%) | ||
There was evidence of external funding in 33.7% (31/92) of clinical research articles and in 100% (4/4) of basic research articles. A breakdown of the types of funding is given in Table 3.
The differences between clinical and basic research were statistically significant (P=.016, Fischer exact test).
Differences Between the Overall Prevalence of External Funding in Spanish Dermatology Departments and Other GroupsExternal funding was present in 36.4% (35/96) of all research articles produced by Spanish dermatology departments. The corresponding percentages for the other groups were 80% for Spanish rheumatology departments (40/50 articles), 44.9% (44/98) for French dermatology departments, and 69.2% (72/104) for British dermatology departments. The differences were statistically significant for Spanish rheumatology departments and British dermatology departments compared to Spanish dermatology departments (P<.001, χ2). The breakdown of percentages by clinical and basic research for each group is shown in Table 3. The percentages for public and industry funding were similar for clinical research. Foundation-type funding was associated with public or industry funding in 38.1% of articles (8/21) published by Spanish dermatology departments. The corresponding figures were 74.2% (23/31) for Spanish rheumatology departments (P=.02, Fischer exact test), 85.7% (18/21) for French dermatology departments (P=.004, Fischer exact test), and 45.3% (29/64) for British dermatology departments (not significant, Fischer exact test).
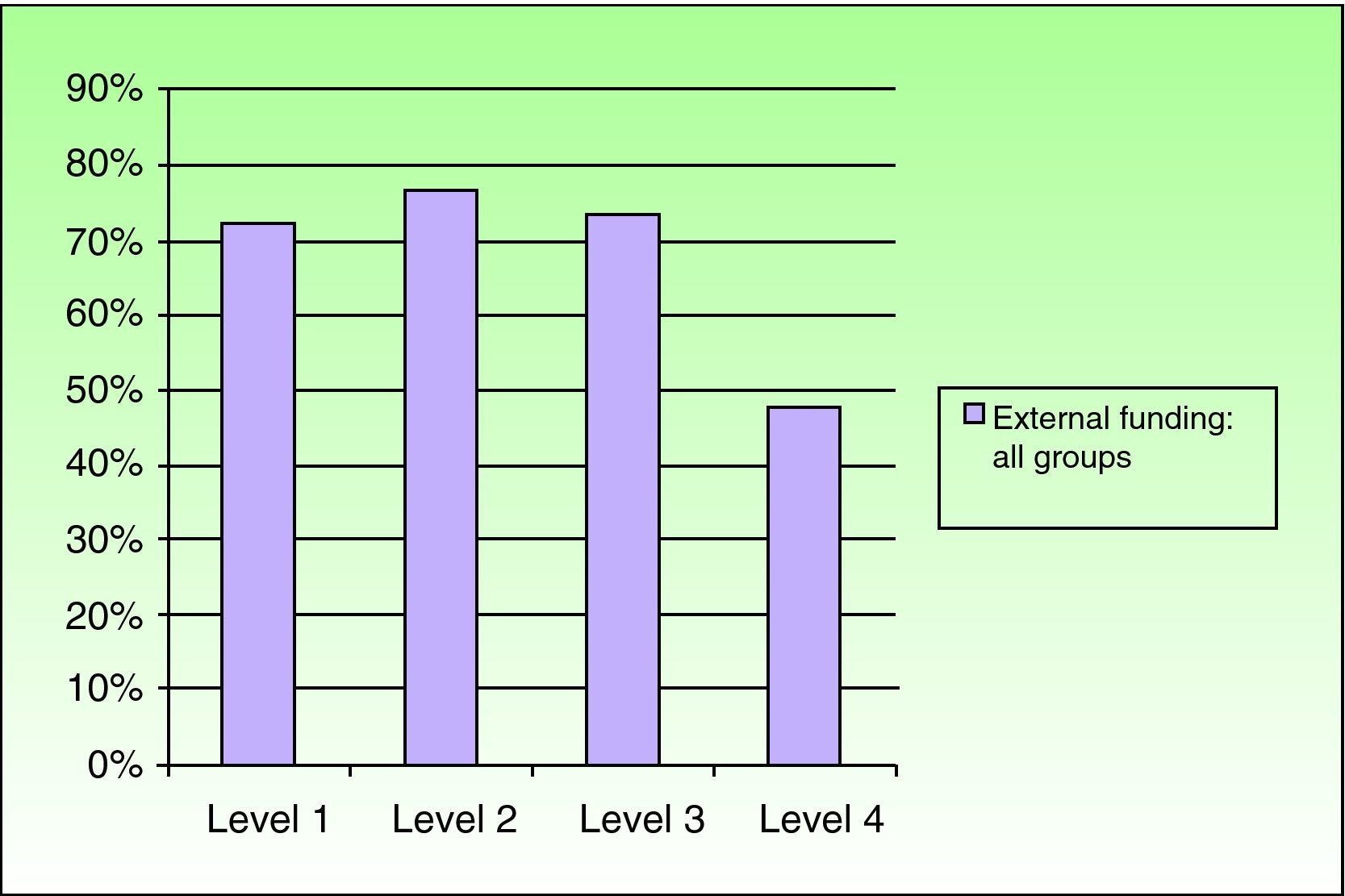
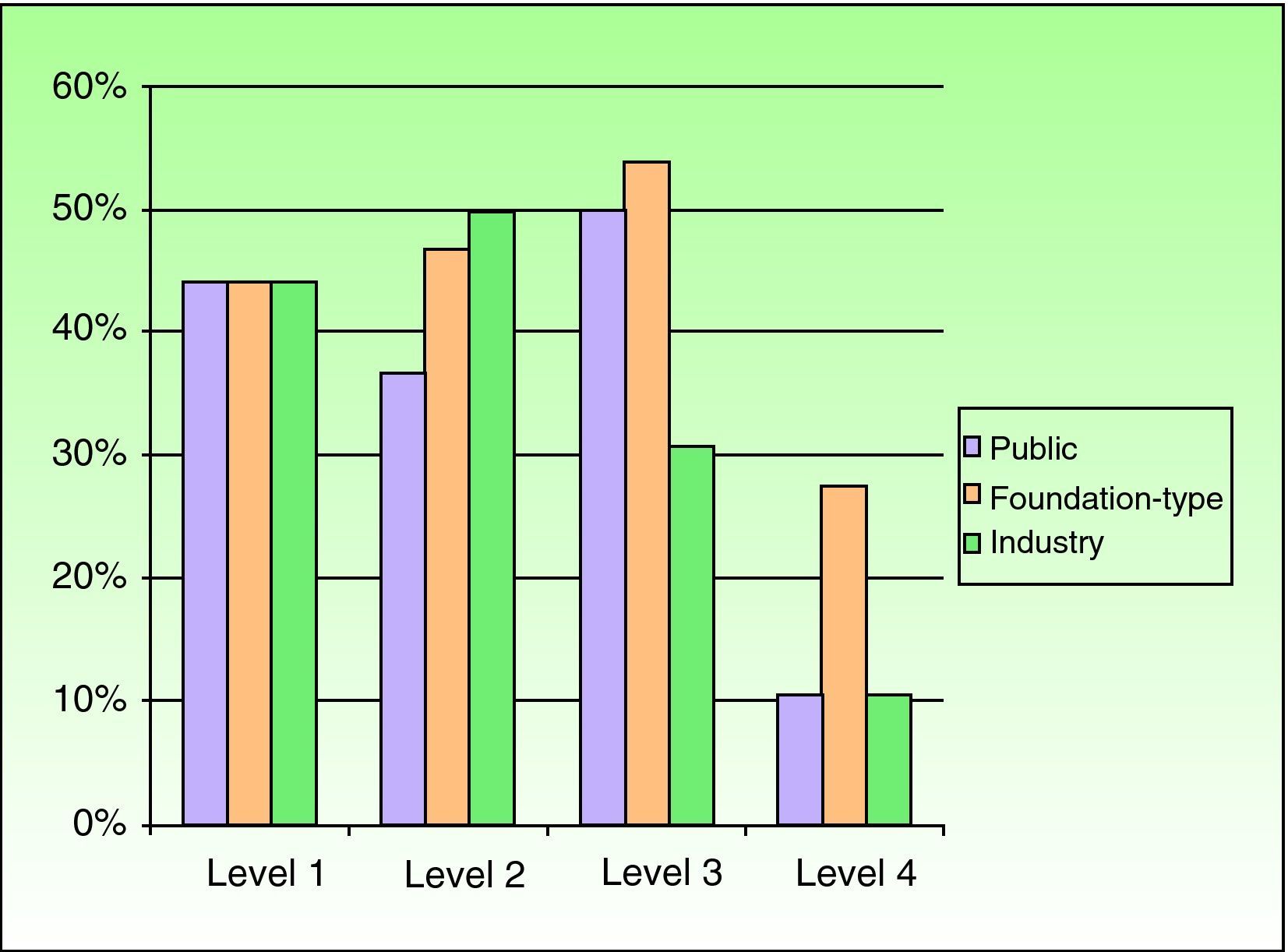
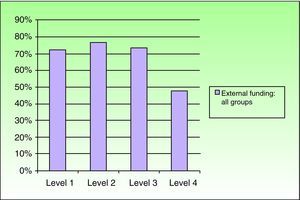
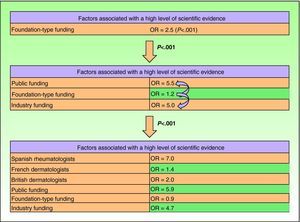
Relationship Between the Funding of Clinical Research Articles and Level of Scientific EvidenceThere was a clear relationship between the presence of external funding and level of scientific evidence of clinical research articles. In the case of articles produced by Spanish dermatology departments, 76.9% (10/13) of the studies with the highest level of evidence (score of ≤3) had received external funding compared to just 26.5% (21/79) of level-4 studies. The differences were statistically significant (P=.003, χ2). The differences persisted when the groups were analyzed together, with a higher prevalence of external funding (overall and specific types) observed in articles with a high level of evidence (P<.001, χ2) (Figs. 2 and 3).
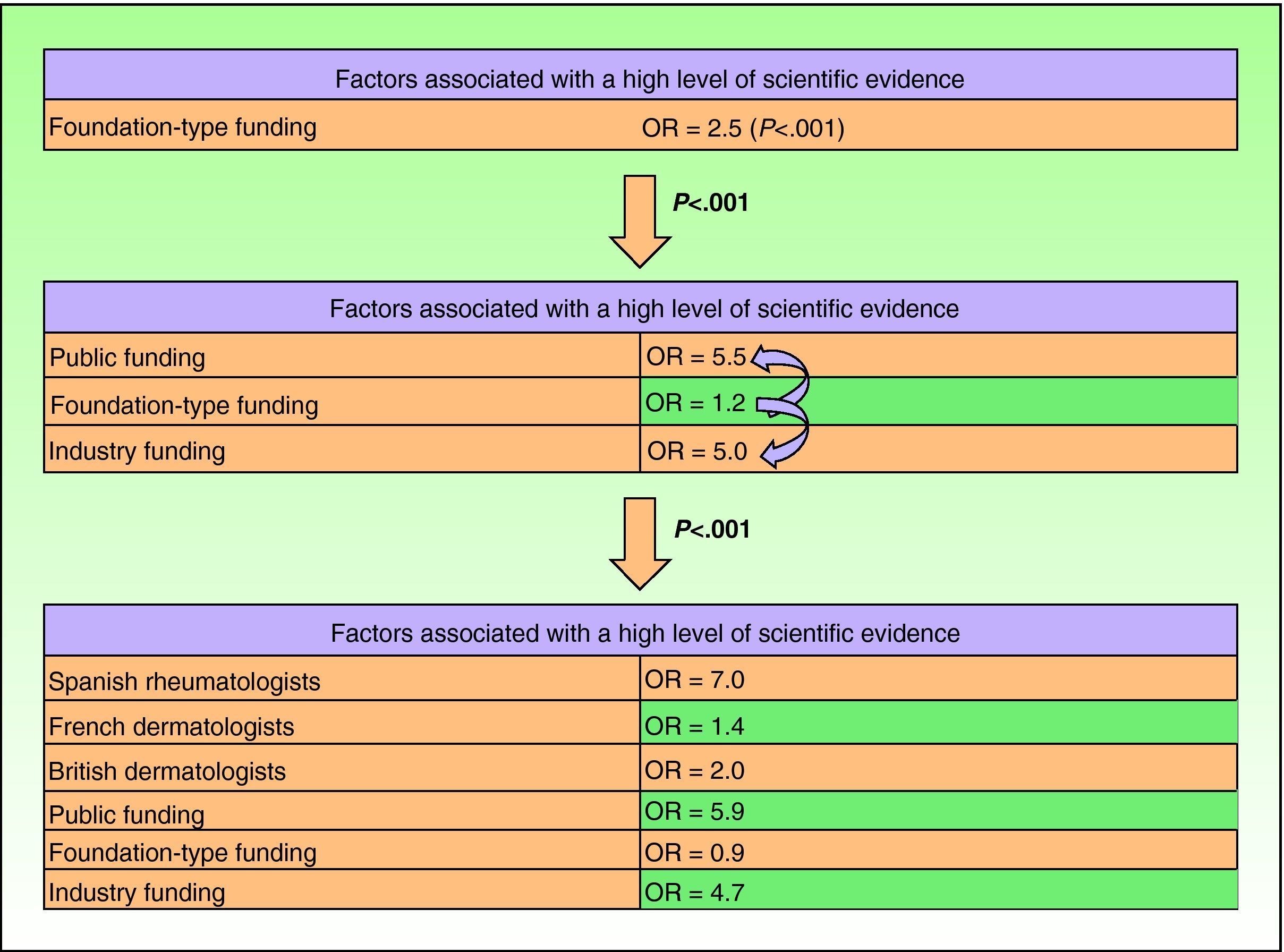
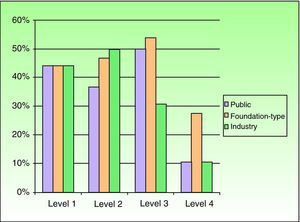
Based on the assumption that the production of articles with a high level of scientific evidence is a desirable goal, the fact that studies with a higher level of evidence were found to have received more funding led us to investigate in more detail the factors that were associated with high levels of evidence. To do this, we performed logistic regression. The first factor included in the model was foundation-type funding, which yielded an odds ratio of 2.5 (P<.001)
On adding public and industry funding, we saw that the model improved significantly (P<.001, likelihood ratio test), indicating that while foundation-type funding exerted an independent effect on quality of scientific evidence, it was also associated with funding by public institutions or industry. The model again improved significantly with the addition of the research group, and this improvement was independent of type of funding. No improvements were seen with the addition of area of research or with interactions between the previous factors.
The final model explained 22% of the variance in level of evidence (pseudo R2) (Fig. 4).
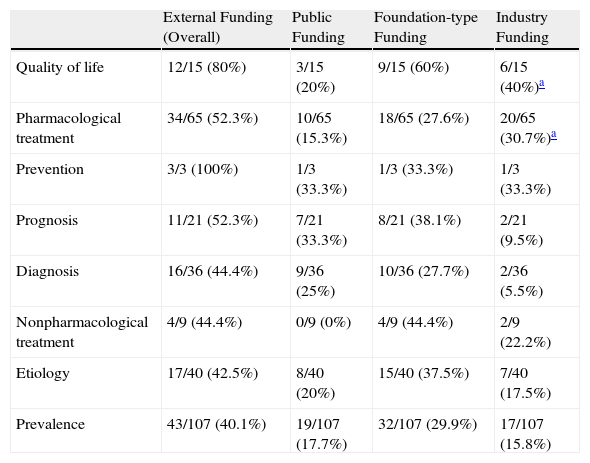
Relationship Between Funding and Area of ResearchOn analyzing the clinical research articles published by Spanish dermatology departments, we observed a higher percentage of funding allocated to studies dealing with quality of life (P=.01, Fisher exact test).
Because of the small number of articles in each research area, we decided to analyze the groups as a whole, and observed a significant association between a higher percentage of funding by industry and research into pharmacological treatment and quality of life. The different areas of research received a comparable proportion of public and foundation-type funding (Table 4).
Funding (Overall and Specific) Allocated to Specific Research Areas for the Group as a Whole.
| External Funding (Overall) | Public Funding | Foundation-type Funding | Industry Funding | |
| Quality of life | 12/15 (80%) | 3/15 (20%) | 9/15 (60%) | 6/15 (40%)a |
| Pharmacological treatment | 34/65 (52.3%) | 10/65 (15.3%) | 18/65 (27.6%) | 20/65 (30.7%)a |
| Prevention | 3/3 (100%) | 1/3 (33.3%) | 1/3 (33.3%) | 1/3 (33.3%) |
| Prognosis | 11/21 (52.3%) | 7/21 (33.3%) | 8/21 (38.1%) | 2/21 (9.5%) |
| Diagnosis | 16/36 (44.4%) | 9/36 (25%) | 10/36 (27.7%) | 2/36 (5.5%) |
| Nonpharmacological treatment | 4/9 (44.4%) | 0/9 (0%) | 4/9 (44.4%) | 2/9 (22.2%) |
| Etiology | 17/40 (42.5%) | 8/40 (20%) | 15/40 (37.5%) | 7/40 (17.5%) |
| Prevalence | 43/107 (40.1%) | 19/107 (17.7%) | 32/107 (29.9%) | 17/107 (15.8%) |
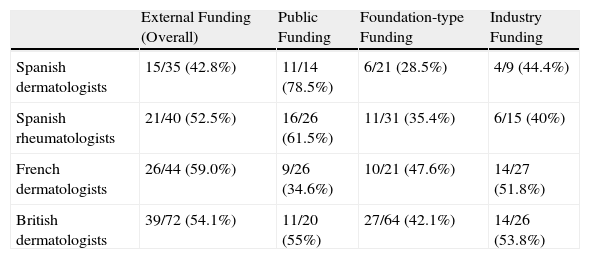
Table 5 shows the percentage of articles that included a specific financial disclosure statement for all groups and types of funding.
Percentage of Funded Articles That Included a Financial Disclosure Statement.
| External Funding (Overall) | Public Funding | Foundation-type Funding | Industry Funding | |
| Spanish dermatologists | 15/35 (42.8%) | 11/14 (78.5%) | 6/21 (28.5%) | 4/9 (44.4%) |
| Spanish rheumatologists | 21/40 (52.5%) | 16/26 (61.5%) | 11/31 (35.4%) | 6/15 (40%) |
| French dermatologists | 26/44 (59.0%) | 9/26 (34.6%) | 10/21 (47.6%) | 14/27 (51.8%) |
| British dermatologists | 39/72 (54.1%) | 11/20 (55%) | 27/64 (42.1%) | 14/26 (53.8%) |
aDenoted by term funded by or similar.
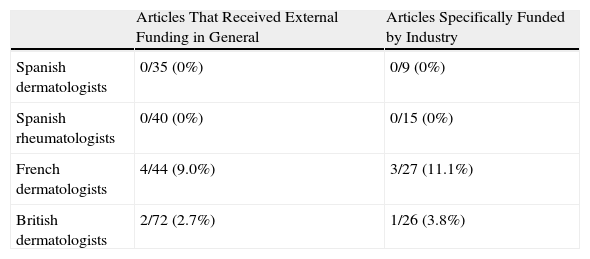
Table 6 shows the percentage of articles that specified the role played by the sponsor for the different groups studied.
Number and Percentage of Funded Articles That Specified the Role of the Sponsor.
| Articles That Received External Funding in General | Articles Specifically Funded by Industry | |
| Spanish dermatologists | 0/35 (0%) | 0/9 (0%) |
| Spanish rheumatologists | 0/40 (0%) | 0/15 (0%) |
| French dermatologists | 4/44 (9.0%) | 3/27 (11.1%) |
| British dermatologists | 2/72 (2.7%) | 1/26 (3.8%) |
This is the first study to describe the different types of funding for research conducted by Spanish dermatology departments and to compare the results with those of similar groups.
It has already been reported that there is inadequate funding for clinical research in Spain. Aibar et al,3 for example, on studying trends in the use of epidemiological designs in clinical research in Spain over a period of 20 years, observed that such designs were uncommon compared to other groups (from other countries) and that funding was uncommon (0% or 23.1% depending on the journal). The authors concluded that the immediate implication was a lack of competitiveness and reduced applicability of Spanish research. Our study confirms that funding is scarce for clinical dermatology research in Spain but it also indicates that not all areas of clinical research are affected, as we detected a higher rate of funding for rheumatology research in Spain.
We also observed that 8.7% of the clinical research studies and 25% of the basic research studies conducted by Spanish dermatology departments had received funding from industry. Other authors have reported higher percentages for other groups and highlighted the importance of such a relationship in terms of conflict of interests.9–11 There is evidence that industry-funded studies produce a higher proportion of favorable results and have better methodological quality than studies with other types of funding.1,2,12 Proportionally, dermatology research in Spain is not more dependent on industry funding than on funding from other sources.
There is a clear relationship between level of scientific evidence, funding, and productivity. Wolf et al,13 for example, detected a relationship between higher impact factors and higher proportions of external funding. In their study, they investigated the percentage of original research articles that had received grants in 3 high-impact journals over 40 years and found a correlation between the impact factor of the journal and the percentage of grant-supported articles published in the journal. Our findings support previous reports of a relationship between a higher percentage of funding and a higher quality of research and group productivity.4 The design of our study did not allow us to establish the direction of this relationship, ie, we were unable to determine whether external funding was allocated to better-quality studies or if the studies were of better quality because they had received funding. We believe that the first option is the most likely because groups become more competitive and secure more external funding from public institutions and industry as they acquire more experience. They are also more likely to benefit from support structures such as foundations, which tend to act as intermediaries for external funding, although these institutions also act as direct sponsors. It is also possible that comparisons, and international comparisons in particular, are hampered by differences in the availability of research funds.
A relationship between area of research and source of funding has been previously reported.14 In our study, we observed a significant relationship between industry funding and studies dealing with pharmacological treatment and quality of life. The relationship with quality of life studies is interesting. We believe that these studies receive a higher proportion of funding from industry because pharmaceutical companies are searching for new outcome measures to support the effectiveness of their drugs, possibly more so when more established or traditional measures have failed to yield sufficiently convincing results.
Full disclosure of conflicts of interest is not the norm.7,12 To resolve this situation, journals have proposed a range of solutions such as the mandatory reporting of all sources of funding and the places of work of the authors, along with adequate registration of clinical trials.1,15 A large proportion of the articles we examined did not adequately comply with financial disclosure guidelines. Editors have also expressed their rejection of contracts which deny researchers the right to examine data independently or to publish a manuscript without first obtaining the consent of the sponsor.8 Despite this, details of the role of the sponsor are given only in a minority of articles. In our analysis, this information was missing in over 90% of the articles analyzed.
Like all research studies, our study has a series of limitations. Our search strategy did not retrieve all the publications by each group. For example, we did not detect articles in which the first author belonged to a department other than a dermatology or rheumatology department (even in cases when all the other authors did) or articles that did not contain an address (mainly letters to the editor). We do not think that this biased our analysis, however, as all journals and groups were affected equally.
There were also some difficulties, described below, in classifying articles but we do not believe that this altered our results as only a few articles were affected.
In some cases it was difficult to distinguish between clinical and basic research, particularly in articles dealing with etiology or diagnostic methods based on the pathogenesis of the disease. These studies were included in one category or the other depending on whether the results of the study had immediate implications for the patient.
There were also some discrepancies when it came to classifying articles by research area, particularly for case series which did not have a clear research question, as this made it difficult to identify the aim of the study. There was no disagreement between the observers, however, when it came to classifying studies on pharmacological treatments.
The classification of types of funding was probably largely correct as the two observers coincided in the vast majority of cases (295/309), and the remaining cases were resolved by a third observer. There were some doubts when it came to distinguishing between public funding and foundation-type funding, but not between public funding and industry funding.
It was also difficult to classify foundations or institutes that provide support to patients, such as the Saint John's Institute of Dermatology or the Fundación Jiménez Díaz. All of these cases were included in the foundation category as we considered their status as a foundation or institute probably increased the likelihood of securing funding for research compared to a public or private hospital.
Another limitation of our study is that our results are based on data provided in the articles and do not reflect undisclosed sources of funding. We believe that this bias was probably reduced by the fact that practically all the journals analyzed have mandatory reporting requirements regarding conflicts of interest and funding.
ConclusionsClinical research conducted by Spanish dermatology departments received a lower proportion of external funding than research performed by the other groups analyzed. Basic research, in contrast, received more funding than clinical research across all groups.
We detected a relationship between a higher proportion of funding and a higher level of scientific evidence, which, in turn, is associated with higher productivity.4 This is probably because research groups that produce better-quality work receive more external funding. Foundations probably act as intermediaries for external funding and provide valuable assistance to these more productive groups.
We also detected a relationship between a higher prevalence of industry funding and studies on pharmacological treatments or quality of life; industry-sponsored quality-of-life studies might reflect the industry's interest in producing new results that support the effectiveness of pharmacological treatments.
A large proportion of articles did not include adequate financial disclosure statements, and information on the role played by sponsors was largely absent.
We should seek to increase our competitiveness in securing funding for clinical research and also improve our compliance with financial disclosure guidelines in dermatology research.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Dr Manuel Pereiro Ferreirós for his assistance in presenting this study towards the awarding of a Master of Advanced Studies (DEA).
Please cite this article as: Batalla A, et al. ¿Quién financia la investigación de los dermatólogos españoles?. Análisis del año 2008 y comparación con otros grupos. Actas Dermosifiliogr. 2011;102:517-526.