Incisional biopsy may not always provide a correct classification of histologic subtypes of basal cell carcinoma (BCC). High-frequency ultrasound (HFUS) imaging of the skin is useful for the diagnosis and management of this tumor.
ObjectivesThe main aim of this study was to compare the diagnostic value of HFUS compared with punch biopsy for the correct classification of histologic subtypes of primary BCC. We also analyzed the influence of tumor size and histologic subtype (single subtype vs. mixed) on the diagnostic yield of HFUS and punch biopsy.
MethodsRetrospective observational study of primary BCCs treated by the Dermatology Department of Hospital Costa del Sol in Marbella, Spain, between october 2013 and may 2014. Surgical excision was preceded by HFUS imaging (DermascanC©, 20-MHz linear probe) and a punch biopsy in all cases. We compared the overall diagnostic yield and accuracy (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) of HFUS and punch biopsy against the gold standard (excisional biopsy with serial sections) for overall and subgroup results.
ResultsWe studied 156 cases. The overall diagnostic yield was 73.7% for HFUS (sensitivity, 74.5%; specificity, 73%) and 79.9% for punch biopsy (sensitivity, 76%; specificity, 82%). In the subgroup analyses, HFUS had a PPV of 93.3% for superficial BCC (vs. 92% for punch biopsy). In the analysis by tumor size, HFUS achieved an overall diagnostic yield of 70.4% for tumors measuring 40mm2 or less and 77.3% for larger tumors; the NPV was 82% in both size groups. Punch biopsy performed better in the diagnosis of small lesions (overall diagnostic yield of 86.4% for lesions ≤40mm2 vs. 72.6% for lesions >40mm2).
ConclusionsHFUS imaging was particularly useful for ruling out infiltrating BCCs, diagnosing simple, superficial BCCs, and correctly classifying BCCs larger than 40mm2.
La biopsia incisional puede fallar en la correcta catalogación de subtipos histológicos de carcinoma basocelular (CBC). La ecografía (ECO) cutánea es una herramienta diagnóstica útil en el diagnóstico y manejo de este tumor.
ObjetivosEl objetivo principal fue evaluar la utilidad diagnóstica de la ECO frente a la biopsia punch en la correcta clasificación del patrón histológico de infiltración de los CBC primarios. Los objetivos secundarios fueron: evaluar si el rendimiento diagnóstico de la ECO frente a la biopsia incisional guardaba relación con el tamaño tumoral y con formas de CBC simples frente a formas mixtas.
MétodosEstudio observacional prospectivo de los casos de CBC primarios atendidos en el Servicio de Dermatología del Hospital Costa del Sol (Marbella) entre octubre de 2013 y mayo de 2014. Previamente a la extirpación quirúrgica se realizó una ECO cutánea (DermascanC©, sonda lineal, 20Mhz) y una biopsia punch. Se valoró el porcentaje de acuerdo absoluto y rendimiento diagnóstico (sensibilidad, especificidad, valor predictivo positivo [VPP] y valor predictivo negativo [VPN]) para resultados globales y parciales entre ECO y punch frente al gold estándar (biopsia escisional por cortes seriados).
ResultadosSe incluyeron 156 casos. La tasa de concordancia diagnóstica global de la ECO fue del 73,7% (sensibilidad: 74,5%, especificidad: 73%) vs. 79,9% (sensibilidad: 76%, especificidad: 82%) para el punch. En el análisis individual destaca para el CBC superficial un VPP para la ECO del 93,3% frente al 92% para el punch. En el análisis por tamaño tumoral la ECO incrementó el porcentaje de acuerdo absoluto del 70,4 al 77,3% (área ≤40mm2 vs. área >40mm2) manteniendo el VPN constante para ambos subgrupos (82%). Para la biopsia punch, el porcentaje de acuerdo absoluto pasó del 86,4 al 72,6%.
ConclusionesLa ECO cutánea muestra una especial utilidad para descartar la presencia de invasividad, para el diagnóstico de formas superficiales simples y para la correcta catalogación de CBC de área mayor a 40mm2.
Basal cell carcinoma (BCC) is the most common cancer in the white population. Patients are increasingly young, and the rise in incidence, together with the enormous associated health care costs,1 calls for new diagnostic techniques that are fast, reliable, and affordable.
Incisional biopsy (generally punch biopsy) is used to confirm a diagnosis of BCC. However, this technique only targets a limited fragment of the tumor and it may fail to correctly classify histologic subtypes. There have been reports of aggressive subtypes being classified as nonaggressive following incisional biopsy. This is more frequent in mixed or large BCCs, where there is a greater chance of missing the most aggressive part of the tumor.2–6 Incorrect classification of histologic subtype can result in inappropriate treatment, increased recurrence rates, and higher costs.2–6
High-frequency ultrasound (HFUS) imaging of the skin is gaining increasing recognition as a first-line diagnostic tool for the diagnosis and management of BCC.7–9 Most studies in this area have highlighted its usefulness for estimating tumor size and mapping out presurgical margins.10–18 However, its role in identifying differential BCC subtype patterns has received considerably less attention and has only been analyzed in small series.9,10,19 Compared with skin biopsy, HFUS has the advantage that it provides an overall vision of the tumor and can therefore potentially distinguish between noninvasive and potentially invasive areas.
The main aim of this study was to compare the diagnostic value of HFUS and punch biopsy for the correct classification of histologic subtypes of primary BCC. A secondary aim was to investigate whether the diagnostic yield of HFUS versus incisional biopsy was related to tumor size and type (simple vs. mixed).
Material and MethodsWe performed a prospective observational study of consecutive cases of primary BCCs referred from primary care to the Dermatology Department at Hospital Costa del Sol in Marbella, Spain between October 2013 and May 2014.
The inclusion criteria were an age of over 18 years and a clinical and dermoscopic diagnosis of a previously untreated primary cutaneous BCC.
A team of independent dermatologists who were not part of the study team enrolled the patients and requested their informed consent. Excluded were patients with primary skin BCC and any of the following: a concomitant disease or allergy to anesthetics that contraindicated surgical excision of the tumor, a mental disorder that would have made it difficult to follow the patient or evaluate results, an indication for Mohs micrographic surgery, or refusal by the patient to provide signed informed consent for participation in the study.
Clinical and epidemiological data (sex, age, tumor size, and location) were recorded for each patient. B-mode HFUS imaging was performed for each lesion using a 20-MHz probe (DermascanC, resolution 60×200μm; Cortex Technology), with recording of tumor size (length, width, depth, and surface area), presence or absence of tumor invasion, and BCC subtype as evidenced by HFUS. Tumor area was calculated using maximum length, width, and depth measurements. Given the difficulty of calculating an exact measurement for tumor area due to the heterogeneous forms, we applied cuboid formulas for subtypes with a cuboid-like form (essentially superficial and infiltrative tumors, but also some nodular tumors) and spherical formulas for essentially nodular/expansive forms. It was accepted that the results were approximate in all cases.
To detect differential BCC patterns detected by HFUS, we consulted the literature in this area.9,10,19,20 The superficial subtype pattern was defined as a flat, heterogeneous, hypoechoic pattern corresponding to a solid tumor with irregular borders without hypoechoic extensions into the underlying dermis; the nodular subtype pattern was defined using similar criteria but applied to an oval/round tumor; the infiltrative subtype pattern was defined as irregular hypoechoic bands extending out from the main tumor mass into the underlying dermis; and the morpheaform subtype pattern was defined by an increase in echogenicity around the main (hypoechoic) tumor mass, corresponding to the characteristic increase in fibrosis seen in these tumors. We found no descriptions of HFUS patterns for micronodular or basosquamous subtypes, but considering the histologic findings described for these tumors, we arbitrarily agreed that a pattern consisting of hypoechoic/anechoic accumulations separated by a hyperechogenic stroma, giving rise to a highly heterogeneous appearance, could be expected findings for the micronodular subtype.
Following HFUS imaging, an independent dermatologist selected an appropriate biopsy site based on clinical and dermoscopic criteria. A 4-mm punch was used for the incisional biopsies. For the histopathologic study, biopsy specimens were fixed in formal, embedded in paraffin, and stained with hematoxylin-eosin. Between 6 and 8 sections were analyzed for each tumor. The suspected diagnosis of BCC was confirmed by histopathologic examination of the biopsy specimen and tumors were classified as invasive or noninvasive. Where possible, histologic subtype was also determined.
Following biopsy, the lesion was surgically excised with 5-mm margins and serial sections were analyzed by a highly experienced dermatopathologist, who examined all the tumors. The tumors were classified into one or more of the following histologic subtypes: superficial, nodular/expansive, infiltrative, micronodular, sclerodermiform, basosquamous, or mixed. Superficial and nodular/expansive subtypes were classified as noninvasive BCCs, while infiltrative, micronodular, sclerodermiform, and basosquamous subtypes were classified as invasive BCCs. Simple forms were defined as tumors with just 1 subtype, while mixed forms were defined as tumors containing 2 or more subtypes. For both the punch and the excisional biopsy, histologic findings were considered to be nonspecific when it was possible to confirm the diagnosis of BCC but not to determine the subtype. Biopsy specimens that were insufficient to establish a histologic diagnosis of BCC were defined as nonevaluable.
We performed a descriptive analysis using measures of central tendency and dispersion for quantitative variables and distribution of frequencies for qualitative variables. We compared the overall diagnostic yield and accuracy (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) of HFUS and punch biopsy against the gold standard (excisional biopsy with serial sections) for overall and subgroup results. Statistical significance was set at a P level of less than .05 and 95% confidence intervals (CIs) were calculated in SPSS version 15.0.1.
ResultsWe analyzed 230 cases of primary BCC, of which 74 (32.17%) were excluded because they did not meet the inclusion criteria. We therefore studied 156 cases in 92 men (59%) and 64 women (41%) with a mean (SD) age of 68 (12) years. The most common general anatomic location for lesions was the head (53.8%), followed by the trunk (28.8%), the cervical region (9.6%), and the arms (7.7%). The cheeks, forehead, and nasal region were the most common sites, accounting for 48.8% of all BCC sites. The mean (SD) clinical dimensions of the lesions were 8.2 (4.5)×6.1 (3)mm, and the mean (SD) surface area was 60.42 (71.02)mm2 (median, 40mm2). The histologic subtypes as determined by the different procedures are shown in Table 1.
Diagnosis by Subtypes for Each Diagnostic Test (n=156).
| HFUS, No. (%) | Punch Biopsy, No. (%) | Excisional Biopsy, No. (%) | |
|---|---|---|---|
| Simple forms | |||
| Superficial BCC | 30 (19.23) | 39 (25) | 45 (28.84) |
| Expansive/nodular BCC | 48 (30.76) | 30 (19.23) | 43 (27.56) |
| Infiltrative BCC | 31 (19.87) | 45 (28.84) | 48 (3.76) |
| Micronodular BCC | 0 (0) | 2 (1.28) | 0 |
| Sclerodermiform BCC | 1 (0.64) | 0 | 0 |
| Mixed forms | |||
| Superficial+nodular | 2 (1.28) | 4 (2.56) | 7 (4.48) |
| Superficial+infiltrative | 8 (5.12) | 5 (3.20) | 5 (3.20) |
| Nodular+infiltrative | 36 (23.07) | 10 (6.41) | 6 (3.84) |
| Mixed infiltrative | 0 | 0 | 0 |
| Not evaluable | 0 | 2 (1.28) | 0 |
| Nonspecific | 0 | 19 (12.17) | 2 (1.28) |
Abbreviations: BCC, basal cell carcinoma; HFUS, high-frequency ultrasound.
The HFUS subtype patterns identified coincide with those described in the literature, and we found no additional differential patterns.
Overall diagnostic yield (compared with the gold standard excisional biopsy) for detecting overall tumor invasion (simple and mixed forms) was 73.7% for HFUS compared with 79.9% for punch biopsy (Table 2A). The respective negative predictive values (NPV) were 82.56% (95% CI, 73.96-91.16) and 84.78% (95% CI, 76.9-92.67).
Contingency Tables.
| A) Contingency Tables for the Detection of Invasion in Simple and Mixed BCCs | |||||||
|---|---|---|---|---|---|---|---|
| HFUS | Excisional Biopsy | Total | Punch Biopsy | Excisional Biopsy | Total | ||
| Invasive | Noninvasive | Invasive | Noninvasive | ||||
| Invasive | 44 | 26 | 70 | Invasive | 45 | 17 | 62 |
| Noninvasive | 15 | 71 | 86 | Noninvasive | 14 | 78 | 92 |
| Total | 59 | 97 | 156 | Total | 59 | 95 | 154 |
| Result (%) | 95% CI | Result (%) | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | 74.58 | 62.62 | 86.53 | Sensitivity | 76.27 | 64.57 | 87.97 |
| Specificity | 73.2 | 63.87 | 82.53 | Specificity | 82.11 | 73.87 | 90.34 |
| Validity index | 73.72 | 66.49 | 80.95 | Validity index | 79.87 | 73.21 | 86.53 |
| PPV | 62.86 | 50.82 | 74.89 | PPV | 72.58 | 60.67 | 84.49 |
| NPV | 82.56 | 73.96 | 91.16 | NPV | 84.78 | 76.9 | 92.67 |
| Prevalence | 37.82 | 29.89 | 45.75 | Prevalence | 38.31 | 30.31 | 46.31 |
| Diagnostic yield: 73.7% | Diagnostic yield: 79.9% | ||||||
| B) Contingency Tables for the Detection of Simple Superficial BCCs | |||||||
|---|---|---|---|---|---|---|---|
| HFUS | Excisional Biopsy | Total | Punch Biopsy | Excisional Biopsy | Total | ||
| Simple Superficial | Not Simple Superficial | Simple Superficial | Not Simple Superficial | ||||
| Simple superficial | 28 | 2 | 30 | Simple superficial | 36 | 3 | 39 |
| Not simple superficial | 17 | 109 | 126 | Not simple superficial | 8 | 107 | 115 |
| Total | 45 | 111 | 156 | Total | 44 | 110 | 154 |
| Result (%) | 95% CI | Result (%) | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | 62.22 | 46.95 | 77.5 | Sensitivity | 81.82 | 69.29 | 94.35 |
| Specificity | 98.2 | 95.27 | 100 | Specificity | 97.27 | 93.77 | 100 |
| Validity index | 87.82 | 82.37 | 93.27 | Validity index | 92.86 | 88.46 | 97.25 |
| PPV | 93.33 | 82.74 | 100 | PPV | 92.31 | 82.66 | 100 |
| NPV | 86.51 | 80.15 | 92.87 | NPV | 93.04 | 87.96 | 98.13 |
| Prevalence | 28.85 | 21.42 | 36.28 | Prevalence | 28.57 | 21.11 | 36.03 |
| Overall diagnostic yield: 87.8% | Overall diagnostic yield: 92.9% | ||||||
| C) Contingency Tables for the Detection of Simple Nodular/Expansive BCCs | |||||||
|---|---|---|---|---|---|---|---|
| HFUS | Excisional Biopsy | Total | Punch Biopsy | Excisional Biopsy | Total | ||
| Simple Nodular | Not Simple Nodular | Simple Nodular | Not Simple Nodular | ||||
| Simple nodular | 28 | 22 | 50 | Simple nodular | 23 | 7 | 30 |
| Not simple nodular | 15 | 91 | 106 | Not simple nodular | 19 | 105 | 124 |
| Total | 43 | 113 | 156 | Total | 42 | 112 | 154 |
| Value, % | 95% CI | Value, % | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | 65.12 | 49.71 | 80.52 | Sensitivity | 54.76 | 38.52 | 71.01 |
| Specificity | 80.53 | 72.79 | 88.27 | Specificity | 93.75 | 88.82 | 98.68 |
| Validity index | 76.28 | 69.29 | 83.28 | Validity index | 83.12 | 76.88 | 89.36 |
| PPV | 56.00 | 41.24 | 70.76 | PPV | 76.67 | 59.87 | 93.47 |
| NPV | 85.85 | 78.74 | 92.96 | NPV | 84.68 | 77.93 | 91.42 |
| Prevalence | 27.56 | 20.23 | 34.90 | Prevalence | 27.27 | 19.91 | 34.63 |
| Overall diagnostic yield: 76.3% | Overall diagnostic yield: 81.1% | ||||||
| D) Contingency Tables for the Detection of Mixed BCCs (Invasive+Noninvasive) | |||||||
|---|---|---|---|---|---|---|---|
| HFUS | Excisional Biopsy | Total | Punch Biopsy | Excisional Biopsy | Total | ||
| Mixed (Invasive+Noninvasive) | Not Mixed (Invasive+Not invasive) | Mixed (Invasive+Noninvasive) | Not Mixed (Invasive+Noninvasive) | ||||
| Mixed (invasive+noninvasive) | 7 | 35 | 42 | Mixed (invasive+noninvasive) | 7 | 8 | 15 |
| Mixed (invasive+noninvasive) | 5 | 109 | 114 | Mixed (invasive+noninvasive) | 5 | 134 | 139 |
| Total | 12 | 144 | 156 | Total | 12 | 142 | 154 |
| Value, % | 95% CI | Value, % | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | 58.33 | 26.27 | 90.39 | Sensitivity | 58.33 | 26.27 | 90.39 |
| Specificity | 75.69 | 68.34 | 83.05 | Specificity | 94.37 | 90.22 | 98.51 |
| Validity index | 74.36 | 67.19 | 81.53 | Validity index | 91.56 | 86.84 | 96.27 |
| PPV | 16.67 | 4.21 | 29.13 | PPV | 46.67 | 18.09 | 75.25 |
| NPV | 95.61 | 91.42 | 99.81 | NPV | 96.4 | 92.95 | 99.86 |
| Prevalence | 7.69 | 3.19 | 12.19 | Prevalence | 7.79 | 3.23 | 12.35 |
| Overall diagnostic yield: 74.4% | Overall diagnostic yield: 91.6% | ||||||
| E) Contingency Tables for the Detection of Mixed BCCs (Superficial+Invasive) | |||||||
|---|---|---|---|---|---|---|---|
| HFUS | Excisional Biopsy | Total | Punch Biopsy | Excisional Biopsy | Total | ||
| Mixed (Superficial+Invasive) | Not Mixed (Superficial+Invasive) | Mixed (Superficial+Invasive) | Not Mixed (Superficial+Invasive) | ||||
| Mixed (superficial+invasive) | 1 | 7 | 8 | Mixed (superficial+invasive) | 2 | 3 | 5 |
| Not mixed (superficial+invasive) | 4 | 144 | 148 | Not mixed (superficial+invasive) | 3 | 146 | 149 |
| Total | 5 | 151 | 156 | Total | 5 | 149 | 154 |
| Value, % | 95% CI | Value, % | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | 20.00 | 0.00 | 65.06 | Sensitivity | 40.00 | 0.00 | 92.94 |
| Specificity | 95.36 | 91.68 | 99.05 | Specificity | 97.99 | 95.40 | 100.00 |
| Validity index | 92.95 | 88.61 | 97.29 | Validity index | 96.10 | 92.72 | 99.48 |
| PPV | 12.50 | 0.00 | 41.67 | PPV | 40.00 | 0.00 | 92.94 |
| NPV | 97.30 | 94.35 | 100.00 | NPV | 97.99 | 95.40 | 100.00 |
| Prevalence | 3.21 | 0.12 | 6.29 | Prevalence | 3.25 | 0.12 | 6.37 |
| Overall diagnostic yield: 92.9% | Overall diagnostic yield: 96.1% | ||||||
| F) Contingency Tables for the Detection of Mixed BCCs (Nodular+Invasive) | |||||||
|---|---|---|---|---|---|---|---|
| HFUS | Excisional Biopsy | Total | Punch Biopsy | Excisional Biopsy | Total | ||
| Mixed (Nodular+Invasive) | Not Mixed (Nodular+Invasive) | Mixed (Nodular+Invasive) | Not Mixed (Nodular+Invasive) | ||||
| Mixed (nodular+invasive) | 4 | 32 | 36 | Mixed (nodular+invasive) | 4 | 6 | 10 |
| Not mixed (nodular+invasive) | 2 | 118 | 120 | Not mixed (nodular+invasive) | 2 | 142 | 144 |
| Total | 6 | 150 | 156 | Total | 6 | 148 | 154 |
| Value, % | 95% CI | Value, % | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | 66.67 | 20.61 | 100.00 | Sensitivity | 66.67 | 20.61 | 100.00 |
| Specificity | 78.67 | 71.78 | 85.56 | Specificity | 95.95 | 92.43 | 99.46 |
| Validity index | 78.21 | 71.41 | 85.00 | Validity index | 94.81 | 90.98 | 98.63 |
| PPV | 11.11 | 0.00 | 22.77 | PPV | 40.00 | 4.64 | 75.36 |
| NPV | 98.33 | 95.63 | 100.00 | NPV | 98.61 | 96.35 | 100.00 |
| Prevalence | 3.85 | 0.51 | 7.18 | Prevalence | 3.90 | 0.52 | 7.28 |
| Overall diagnostic yield: 78,2% | Overall diagnostic yield: 94,8% | ||||||
Abbreviations: BCC, basal cell carcinoma; NPV, negative predictive power; PPV, positive predictive power.
In the individual analysis by BCC subtype (Table 2), HFUS had a diagnostic yield of 87.8% (positive predictive value [PPV], 93.33% [95% CI, 82.4-100]), while punch biopsy had a yield of 92.9% (PPV, 92.31% [95% CI, 82.66-100]) (Table 2A).
Compared with excisional biopsy, the respective diagnostic yields for HFUS and punch biopsy for mixed forms were 74.4% and 91.6% (Table 2D). Both procedures had a particularly high NPV for mixed forms, whether superficial and infiltrative or nodular and invasive (Table 2E, F).
The results for the analysis by tumor size are shown in Table 3. HFUS achieved a diagnostic yield of 70.4% for tumors measuring 40mm2 or less and 77.3% for those measuring over 40mm2. The NPV was very similar in both groups: 82.35% (95% CI, 70.91-93.80) for HFUS and 82.86% (95% CI, 68.94-96.77) for punch biopsy. The overall diagnostic yield for punch biopsy fell from 86.4% for tumors measuring 40mm2 or less to 72.6% for larger tumors. The PPV and NPV also decreased. In the latter case, the reduction (91.07% [95% CI, 82.71-99.43] to 75% [95% CI, 59.47-90.53]) was significant.
Contingency Tables for Analysis by Tumor Size.
| A) Contingency Table for the Detection of Invasion in Tumors ≤40mm2 | |||||||
|---|---|---|---|---|---|---|---|
| HFUS | Excisional Biopsy | Total | Punch Biopsy | Excisional Biopsy | Total | ||
| Invasive | Noninvasive | Invasive | Noninvasive | ||||
| Invasive | 15 | 15 | 30 | Invasive | 19 | 6 | 25 |
| Noninvasive | 9 | 42 | 51 | Noninvasive | 5 | 51 | 56 |
| Total | 24 | 57 | 81 | Total | 24 | 57 | 81 |
| Value, % | 95% CI | Value, % | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | 62.50 | 41.05 | 83.95 | Sensitivity | 79.17 | 60.84 | 97.50 |
| Specificity | 73.68 | 61.38 | 85.99 | Specificity | 89.47 | 80.63 | 98.32 |
| Validity index | 70.37 | 59.81 | 80.93 | Validity index | 86.42 | 78.34 | 94.50 |
| PPV | 50.00 | 30.44 | 69.56 | PPV | 76.00 | 57.26 | 94.74 |
| NPV | 82.35 | 70.91 | 93.80 | NPV | 91.07 | 82.71 | 99.43 |
| Prevalence | 29.63 | 19.07 | 40.19 | Prevalence | 29.63 | 19.07 | 40.19 |
| Overall diagnostic yield: 70.4% | Overall diagnostic yield: 86.4% | ||||||
| B) Contingency Table for the Detection of Invasion in BCCs >40mm2 | |||||||
|---|---|---|---|---|---|---|---|
| HFUS | Excisional Biopsy | Total | Punch Biopsy | Excisional Biopsy | Total | ||
| Invasive | Noninvasive | Invasive | Noninvasive | ||||
| Invasive | 29 | 11 | 40 | Invasive | 26 | 11 | 37 |
| Noninvasive | 6 | 29 | 35 | Noninvasive | 9 | 27 | 36 |
| Total | 35 | 40 | 75 | Total | 35 | 38 | 73 |
| Value, % | 95% CI | Value, % | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | 82.86 | 68.94 | 96.77 | Sensitivity | 74.29 | 58.38 | 90.19 |
| Specificity | 72.50 | 57.41 | 87.59 | Specificity | 71.05 | 55.32 | 86.79 |
| Validity index | 77.33 | 67.19 | 87.48 | Validity index | 72.60 | 61.69 | 83.52 |
| PPV | 72.50 | 57.41 | 87.59 | PPV | 70.27 | 54.19 | 86.35 |
| NPV | 82.86 | 68.94 | 96.77 | NPV | 75.00 | 59.47 | 90.53 |
| Prevalence | 46.67 | 34.71 | 58.62 | Prevalence | 47.95 | 35.80 | 60.09 |
| Overall diagnostic yield: 77,3% | Overall diagnostic yield: 72,6% | ||||||
Abbreviations: BCC, basal cell carcinoma; NPV, negative predictive power; PPV, positive predictive power.
Excisional surgery is still considered to be the most effective treatment for BCC, as it permits the analysis of surgical margins following excision; accordingly, it is also associated with the lowest recurrence rates.6,21,22 In recent years, however, nonsurgical treatments (mainly photodynamic therapy and topical imiquimod 5%) have been increasingly used to treat noninvasive and low-risk BCCs as well as BCCs for which surgery is contraindicated or rejected by the patient.21 While these treatments are associated with lower morbidity, lower costs, and better cosmetic results,6 they are also linked to higher recurrence, with rates of between 5% and 30% depending on the series and the technique.6,22 Correctly identifying thus which tumors are candidates for nonsurgical treatment (essentially noninvasive tumors) is therefore key to reducing subsequent recurrence.
Clinical and dermoscopic examination is very useful for establishing a pretreatment diagnosis. However, the diagnosis must then be confirmed by incisional biopsy, the current gold standard. Incisional biopsy also allows for the determination of histologic subtype, which is crucial for characterizing the aggressiveness of the tumor and planning the necessary treatment.21 In addition, when tumors are treated with a nonsurgical approach, the full extent of the tumor is not analyzed histologically, and at most, the only information available is that provided by a biopsy of a small portion. This highlights the importance of establishing a correct diagnosis prior to treatment.
Punch biopsy has been reported to have serious shortcomings when it comes to accurately classifying aggressive and nonaggressive forms of BCC.4 Correct classification rates reported for incisional biopsy in large series range from 51% to 83% according to the series,2–4 while underdiagnosis rates for aggressive forms range between 7% and 39%.2–4
In the current series, punch biopsy had a sensitivity of 76.27% for detecting overall tumor invasion (simple and mixed forms) (Table 2A). This means that approximately 24% of BCCs that eventually turned out to be invasive were initially classified as nonaggressive (Fig. 1). This figure coincides with rates reported in the literature cited above. The sensitivity rate for HFUS was similar, at 74.8%, but it had a high NPV (82.56% vs. 84.78% for punch biopsy) (Table 3). This NPV indicates that the particular usefulness of HFUS lies not in its ability to detect invasive forms but rather in its ability to correctly detect noninvasive forms.
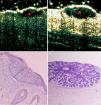
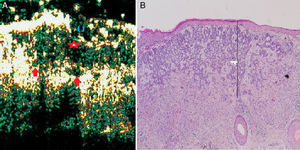
Infiltrative basal cell carcinoma. A, B-mode (20Mhz) cross-section image. Note the heterogeneous subepidermal hypoechoic image (red star) with irregular albeit well-defined borders and numerous hypoechoic extensions into the underlying dermis (red arrows), indicating infiltration. The lesion is also ulcerated, as shown by the interruption in the underlying epidermis (U). B, Histologic image of the tumor (hematoxylin-eosin, original magnification ×4).
On analyzing the subgroup of superficial BCCs (Table 2B), we observed a PPV of 93.33% for HFUS, which is similar to that observed for punch biopsy. In other words, HFUS would correctly identify over 9 of every 10 superficial BCCs analyzed by showing a flat, well-defined subepidermal hypoechoic pattern, without hypoechoic prolongations extending into the underlying dermis (Fig. 2). Like biopsy thus, it would produce an adequate histologic classification for determining a noninvasive treatment.
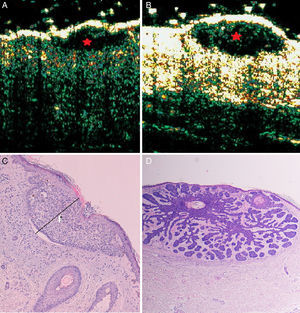
Superficial, nodular basal cell carcinoma. B-mode (20Mhz) longitudinal section. A, Heterogeneous subepidermal anechoic flat image with irregular but well-defined borders (red star), corresponding to a superficial subtype. B, Similar ultrasound appearance but with an oval shape, corresponding to a nodular subtype (red star). C and D, Histologic images of the tumors (hematoxylin-eosin×4).
As described in the literature, punch biopsy loses sensitivity when used to determine histologic subtypes in mixed and larger tumors, where there is obviously a greater chance of missing the invasive part of the tumor.2 Although the diagnostic yield of punch biopsy with respect to the gold standard excisional biopsy is relatively good for BCCs containing a single subtype, it is considerably less so for mixed BCCs (83% vs. 37%).2 Correct identification of aggressive forms may thus be difficult, as a high percentage of BCCs (18% to >50% according to the series) have more than 1 histologic variant.6 Our series shows that punch biopsy performed better at identifying invasion in simple forms than in mixed forms (75% vs. 58.33%), although the difference was not significant (Table 2D). The corresponding sensitivity rates for HFUS were 47.92% for simple invasive forms and 58.33% for mixed forms. This latter rate was identical to that observed for punch biopsy and both techniques had a high NPV (around 97%). In other words, both HFUS and punch biopsy are better at detecting invasion in mixed tumors than in simple tumors (Fig. 3) and HFUS is at least as good as punch biopsy in this case. It is important to note that mixed BCCs accounted for just 11.68% of the tumors analyzed in the present series. This rate is lower than that described for other series,4,6 and possibly explains why the differences observed were not significant.
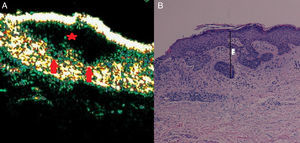
Mixed basal cell carcinoma (superficial and infiltrative). A, B-mode (20Mhz) longitudinal image. Note the anechoic flat heterogeneous subepidermal image with irregular but well-defined borders (red star), in addition to 2 extensions into the underlying dermis on the far right (red arrows), corresponding to foci of infiltration. B, Histologic image of the tumor (hematoxylin-eosin, original magnification ×4).
The results for the diagnostic yield of both HFUS and punch biopsy in relation to tumor size are also interesting (Table 3). HFUS had a diagnostic yield of 70.4% for tumors measuring 40mm2 or less and of 77.3% for larger tumors. The NPV for both subgroups was similar, at around 82%. The yield for punch biopsy, however, fell from 86.4% to 72.6% when used to analyze tumors over 40m2. A lower PPV and NPV were also observed in such cases, and the reduction for NPV was significant (91.07% to 75%). These results support previous reports of a lower diagnostic yield for punch biopsy for larger tumors.2 HFUS in turn correctly detected invasion in 29 of the 35 invasive BCCs over 40mm2 (sensitivity of 82.86%). The corresponding sensitivity for punch biopsy was 74.29% (26/35 cases), showing that this technique failed to detect 3 invasive BCCs that HFUS successfully identified. These 3 tumors could therefore have been incorrectly classified and prescribed an inappropriate nonaggressive treatment. Our findings suggest that HFUS performs slightly better in the evaluation of larger tumors, and this would be logical considering that it provides a real-time image of the full extent of the tumor, whereas punch biopsy analyzes just a part.
Our study has some limitations. First, we were unable to determine how HFUS performs with morpheaform, micronodular, and basosquamous BCCs, as these were missing from our sample. Second, we did not perform a Doppler study and were thus unable to analyze its ability to detect different subtypes. Finally, as previously mentioned, mixed BCCs accounted for just a small proportion of the tumors in our series.
ConclusionsHFUS is a useful, noninvasive diagnostic tool for the real-time classification of invasive and noninvasive BCCs. It proved particularly useful for ruling out invasion (especially in simple forms) and correctly classifying histologic subtypes in tumors over 40 mm2. Based on our findings, we call for the protocol-based use of HFUS (together with punch biopsy) for the evaluation of BCCs, particularly before prescribing a nonsurgical treatment.
Ethical DisclosuresProtection of humans and animalsThe authors declare that the procedures followed complied with the ethical standards of the corresponding human experimentation committee and the World Medical Association and with the principles of the Declaration of Helsinki.
Confidentiality of dataThe authors declare that no private patient data appear in this article.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank the physicians, nurses, and assistants from the Dermatology Department of Hospital of Hospital Costa del Sol in Marbella, Spain for their invaluable help in this study. Without their help, this study would not have been possible.
Please cite this article as: Hernández-Ibáñez C, Blazquez-Sánchez N, Aguilar-Bernier M, Fúnez-Liébana R, Rivas-Ruiz F, de Troya-Martín M. Utilidad de la ecografía cutánea en la clasificación de subtipos de los carcinomas basocelulares primarios. Actas Dermosifiliogr. 2017;108:42–51.