Merkel cell carcinoma (MCC) is a rare, highly aggressive tumor, and local or regional disease recurrence is common, as is metastasis. MCC usually develops in sun-exposed skin in patients of advanced age. Its incidence has risen 4-fold in recent decades as the population has aged and immunohistochemical techniques have led to more diagnoses. The pathogenesis of MCC remains unclear but UV radiation, immunosuppression, and the presence of Merkel cell polyomavirus in the tumor genome seem to play key roles. This review seeks to update our understanding of the epidemiology, etiology, pathogenesis, and clinical features of MCC. We also review histologic and immunohistochemical features required for diagnosis. MCC staging is discussed, given its great importance in establishing a prognosis for these patients.
El carcinoma de células de Merkel (CCM) es un tumor poco frecuente, con un curso evolutivo muy agresivo, que con frecuencia origina recidivas locorregionales y metástasis. Asienta predominantemente en zonas fotoexpuestas en pacientes ancianos. Su incidencia se ha cuadriplicado en las últimas décadas debido al envejecimiento de la población y a un mayor diagnóstico gracias al uso de técnicas inmunohistoquímicas. La patogénesis del CCM no está clara, pero la radiación ultravioleta, la inmunosupresión y la presencia del poliomavirus MCPyV en el genoma del tumor parecen desempeñar un papel fundamental en el desarrollo de esta neoplasia.
El objetivo de este artículo es realizar una revisión actualizada sobre los diferentes aspectos de la epidemiología, la etiopatogenia y la clínica del CCM. A su vez, detallamos los aspectos histológicos e inmunohistoquímicos necesarios para su diagnóstico y revisamos la estadificación por su importante trascendencia en el pronóstico de estos pacientes.
Merkel cell carcinoma (MCC), also known as primary cutaneous neuroendocrine carcinoma, is an rare skin tumor that affects patients of advanced age. MCC is highly aggressive, and local and regional spread and metastasis are very common. Because the incidence of MCC has risen and a virus has been implicated in its pathogenesis, interest has increased in recent years.
This review aims to update our understanding of the epidemiology, etiology, pathogenesis, and clinical features of MCC. We review the histologic and immunohistochemical findings that lead to diagnosis and discuss the current staging system of the American Joint Committee on Cancer.
HistoryThe author of the first description of a MCC tumor, which appeared in 1972, was Toker,1 who used the term trabecular carcinoma of the skin to suggest the possibility of an origin in glandular tissue. An ultrastructural study by Tang and Toker2 that appeared 6 years later demonstrated the presence of electron-dense granules in the cytoplasm of tumor cells. They proposed a neuroendocrine origin similar to that of Merkel cells in the epidermis. In fact debate on the cellular origin of these tumors continues, accounting for the proliferation of nomenclature used over time: cutaneous APUdoma, primary small cell carcinoma of the skin, anaplastic carcinoma of the skin, endocrine carcinoma of the skin, Merkeloma, primary cutaneous neuroendocrine carcinoma, and MCC.3–5 These terms reflect neuroendocrine cell differentiation, the cell morphology observed, and a histologic similarity to small cell lung cancer.
The term cutaneous neuroendocrine carcinoma is the one that perhaps best reflects the immunohistochemical and ultrastructural features of these tumors, but the most commonly used and universally accepted name in the literature is MCC.
EpidemiologyMCC is a rare but characteristically aggressive tumor whose mortality rate is twice that of melanoma.6 Less than 1% of malignant skin tumors are MCCs,7 but it is the third most frequent cause of death from skin cancer after melanoma and squamous cell carcinoma.8
The incidence of MCC in Spain is unknown at this time. However, it has risen 3-fold in recent decades in the United States, from 0.15 per 100000 population in 1981 to 1.44 per 100000 population in 2011.9,10 The increase is attributable to greater awareness of the disease among dermatologists and pathologists; greater ease of diagnosis thanks to immunohistochemical techniques; and increased population risk due to UV light exposure, aging, and immunosuppression.11
MCC affects mainly white populations but also appears exceptionally in black persons. The prevalence is somewhat higher in men than women in some professions (1.4:1) but no differences between the sexes are seen in others.7,12
MCC is diagnosed more often in persons of advanced age, and the incidence peaks between the seventh and eighth decades of life.13 The mean age at diagnosis is 75 years. Only 5% of patients are under the age of 50 years, when the tumor is usually associated with some form of immunosuppression.12 Nonetheless, cases have been described even in children, the youngest being 7 years old.14
Etiopathogenesis and Populations at RiskMCC affects light-skinned individuals (phototypes I-III) on sun-exposed parts of the body and is associated with other skin tumors. These associations point to an etiopathogenic role for UV light,15,16 but MCC has also been seen in patients who have undergone radiation or psoralen-UV-A therapy, in individuals exposed to arsenic,17 and in skin areas affected by erythema ab igne.18 MCC patients also develop other skin tumors induced by UV radiation (e.g., basal cell or squamous cell carcinomas and melanoma) that may appear either before or after MCC. Patients with melanoma are at 3-fold higher risk of MCC than the general population.19
The frequency is higher in immunocompromised individuals,12,20 especially after heart21 or kidney22 transplants; during immunosuppressant treatment with azathioprine, ciclosporin, cyclophosphamide, and mTOR (target of rapamycin) inhibitors23; and in persons living with human immunodeficiency virus (HIV) infection.24 Therefore, immune compromise is a risk factor for MCC, which also develops at younger ages and follows a more aggressive course in this context. MCC appears about 10 years after a transplant on average, and the ratio of melanoma to MCC is 1:6 in transplanted patients and 1:65 in the general population.
Risk for MCC is 13-fold higher in HIV-infected individuals than in the general population. A study of 14 HIV-infected patients with MCC found that most were males and on average younger (49 years old) at diagnosis than patients with MCC and no HIV infection (70 years old).25 Other differences between the behavior of MCC in HIV-infected and noninfected persons are that the tumor develops in unexposed areas in the presence of HIV (35% on the buttocks) and that mean survival is shorter (less than 19 months after diagnosis).
Occasionally MCC has been described in association with blood diseases26 (chronic lymphocytic leukemia, non-Hodgkin's lymphoma, and multiple myeloma); with solid tumors27 (of the lung, pancreas, prostate, breast, or colon); or with autoimmune diseases (rheumatoid arthritis, ankylosing spondylitis, Crohn disease, and inflammatory bowel disease).28,29 Within these groups of diseases, chronic lymphocytic leukemia is the condition most often associated with MCC. There have been sporadic reports of a link between MCC and congenital ectodermal dysplasia and Cowden syndrome.30
The most important discovery related to pathogenesis came in 2008 with the description of the Merkel cell polyomavirus (MCPyV) in the genome of MCC tumor cells. Fengetal.31 found MCPyV expression in 80% (8 of the 10 MCCs they analyzed) but in only 8% of control samples from various organs and in 16% of control samples of healthy skin. Later studies confirmed MCPyV expression in about 80% of MCCs. However, it has been suggested that variation in the incidence of expression may be related to geographic differences.
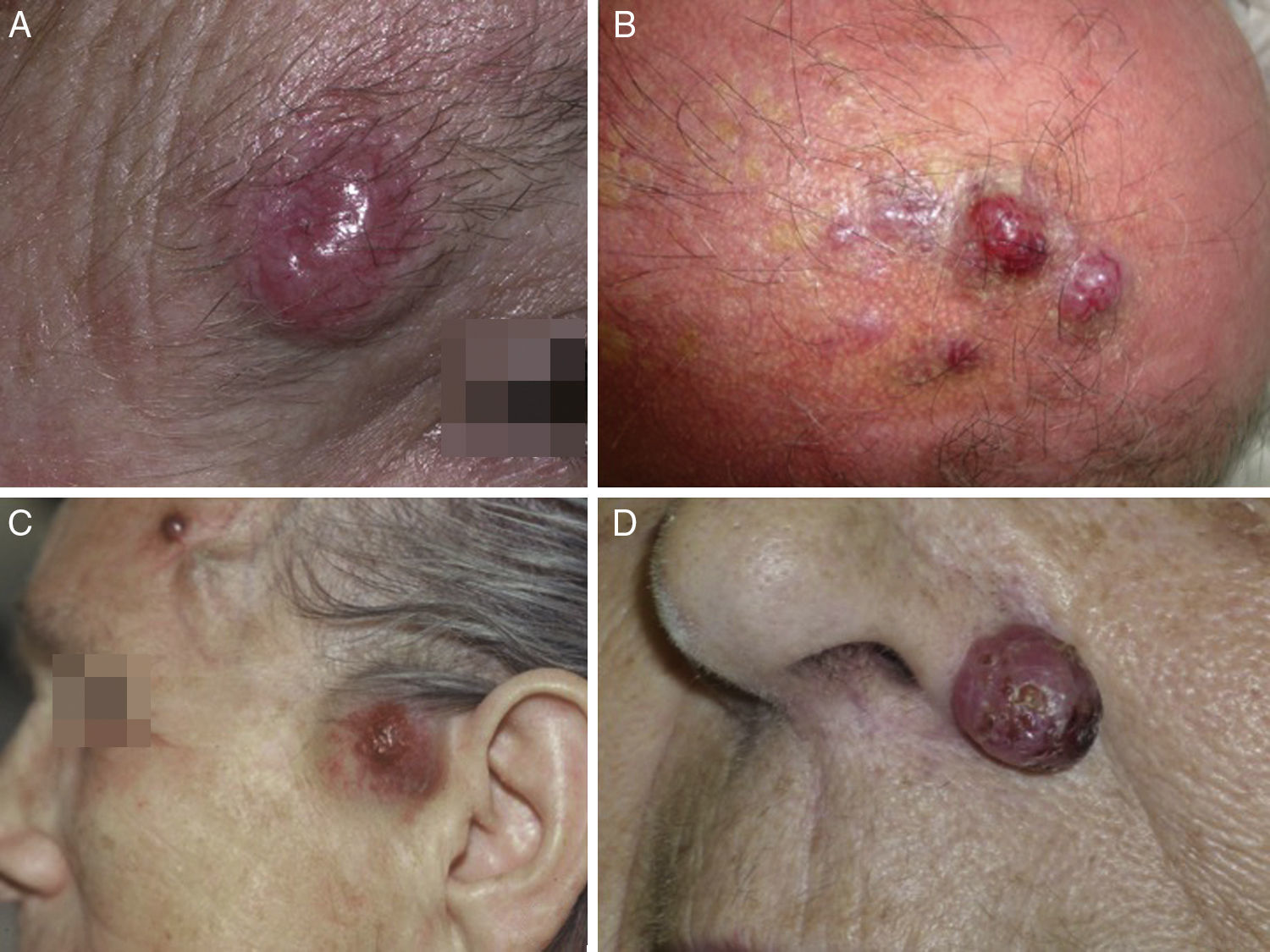
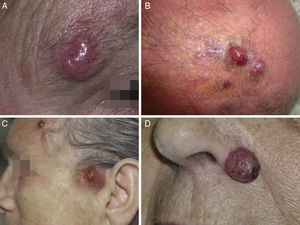
Clinical FeaturesMCC usually presents as a single firm, well-defined nodule that is erythematous, or violaceous in color, and mobile with respect to underlying tissue. The surface is soft, smooth and shiny, rarely ulcerated, and often marked by telangiectasis32,33 (Fig. 1A). MCC may present as a single dermal or subcutaneous plaque or as a multinodular lesion (Fig. 1B). Less often a multifocal or disseminated lesion (Fig. 1C) might be found.34–36 Pediculated tumors (Fig. 1D) have also been described.37
The tumor is asymptomatic and the patient usually consults a physician because of rapid growth over a period of weeks or months.38 The average tumor size on diagnosis is 2 to 4cm,5,33,39–41 and large lesions sometimes have an erythematous inflammatory halo. Histology of the halo area does not show an inflammatory infiltrate, however, but does reveal vascular invasion. Therefore, a halo sign suggests a poor prognosis.
The tumor usually develops in a sun-exposed area but can grow in any skin or mucosal tissue. The head or neck is the most common site, accounting for around half; the next most common sites are the extremities (40%) and trunk and buttocks (10%).32,35,42,43 A periorbital location is very frequent (10%) (Fig. 1A), and a tumor at this site may be confused with a benign cyst or lipid granuloma. MCC has very infrequently been described in unexposed areas, such as the genitals44,45 or perianal and oropharyngeal46 mucosal tissue.
MCC has been found in lymph nodes in the absence of a primary skin tumor.47 Primary lymph node MCCs have been associated with a better prognosis than primary skin MCCs with affected lymph nodes.48 Spontaneous partial or total regression of these tumors after biopsy has also been reported.49,50
In some cases, MCC in situ or a small incipient baby Merkel tumor is diagnosed while a patient is being followed for basal cell or squamous cell carcinoma.51–54 This tumor may also be an incidental finding on inspection of biopsy material. Prognosis in such cases is better.
If a lesion initially appears to be benign, treatment of MCC may be delayed, leading clinicians to use of the mnemonic AEIOU to recall relevant features: asymptomatic, expanding rapidly, immunosuppressed, older than 50 years, and UV exposed. The goal is to avoid delaying diagnosis. MCC is initially suspected in only about 1% of cases.55
Differential clinical diagnoses are highly varied, including both benign lesions (epidermal cyst, dermatofibroma, fibroma, angioma, and lipid granuloma) and malignant ones (squamous cell carcinoma, amelanotic melanoma, cutaneous metastasis of other tumors, lymphoma, basal cell carcinoma, keratoacanthoma, and adnexal tumors).54
Characteristic dermoscopic features of MCC have not been established, but there are published descriptions of the presence of unorganized milky-red areas and arborizing vessels, features that raise suspicion of malignancy in other lesions besides MCC.56,57
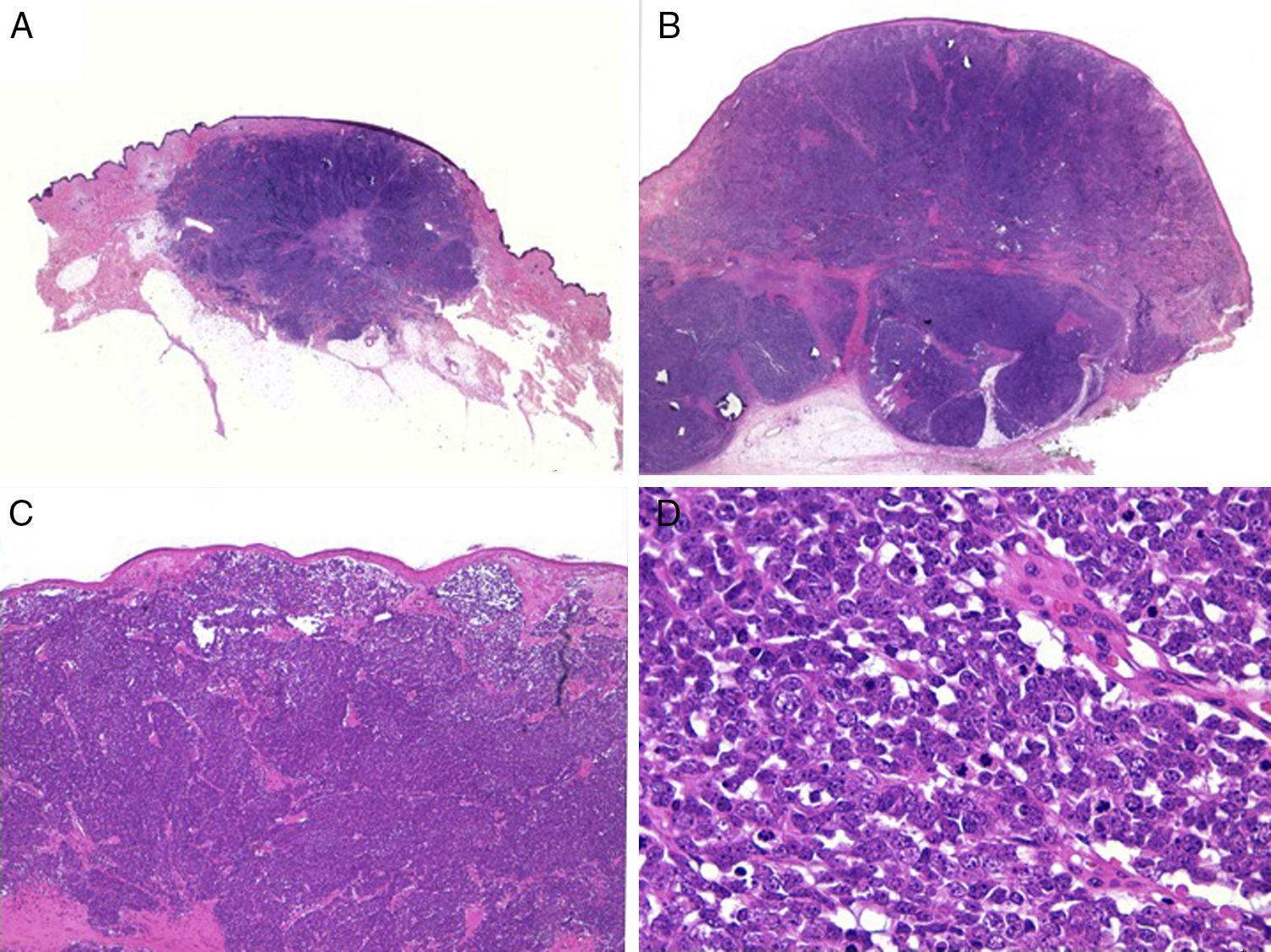
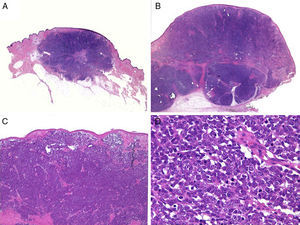
Histologic FeaturesMacroscopically, MCCs are well demarcated, unencapsulated, firm, and homogeneous. Microscopically, they are dermal lesions composed of 2 types of round, basophilic, monomorphic cells whose large vesicular nuclei have finely granular (salt-and-pepper) chromatin condensation58 (Fig. 2). It is common to see necrotic areas, pyknotic nuclei, and an abundance of mitotic figures (Fig. 2D). Vascular invasion and an abundant inflammatory infiltrate of lymphocytes and plasma cells surrounding the tumor are other common findings. An infiltrate is sometimes also found inside the tumor.
Histology. A and B, Panoramic view of a Merkel cell carcinoma. The tumor is well defined, unencapsulated, and nodular; hematoxylin-eosin, original magnification ×10. C, Localized tumor in the dermis. The cells are round, basophilic, and monomorphic; hematoxylin-eosin, original magnification ×100. D, Round, blue cells with scant cytoplasm, and finely granular chromatin condensation. Numerous mitotic figures are present; hematoxylin-eosin, original magnification ×400.
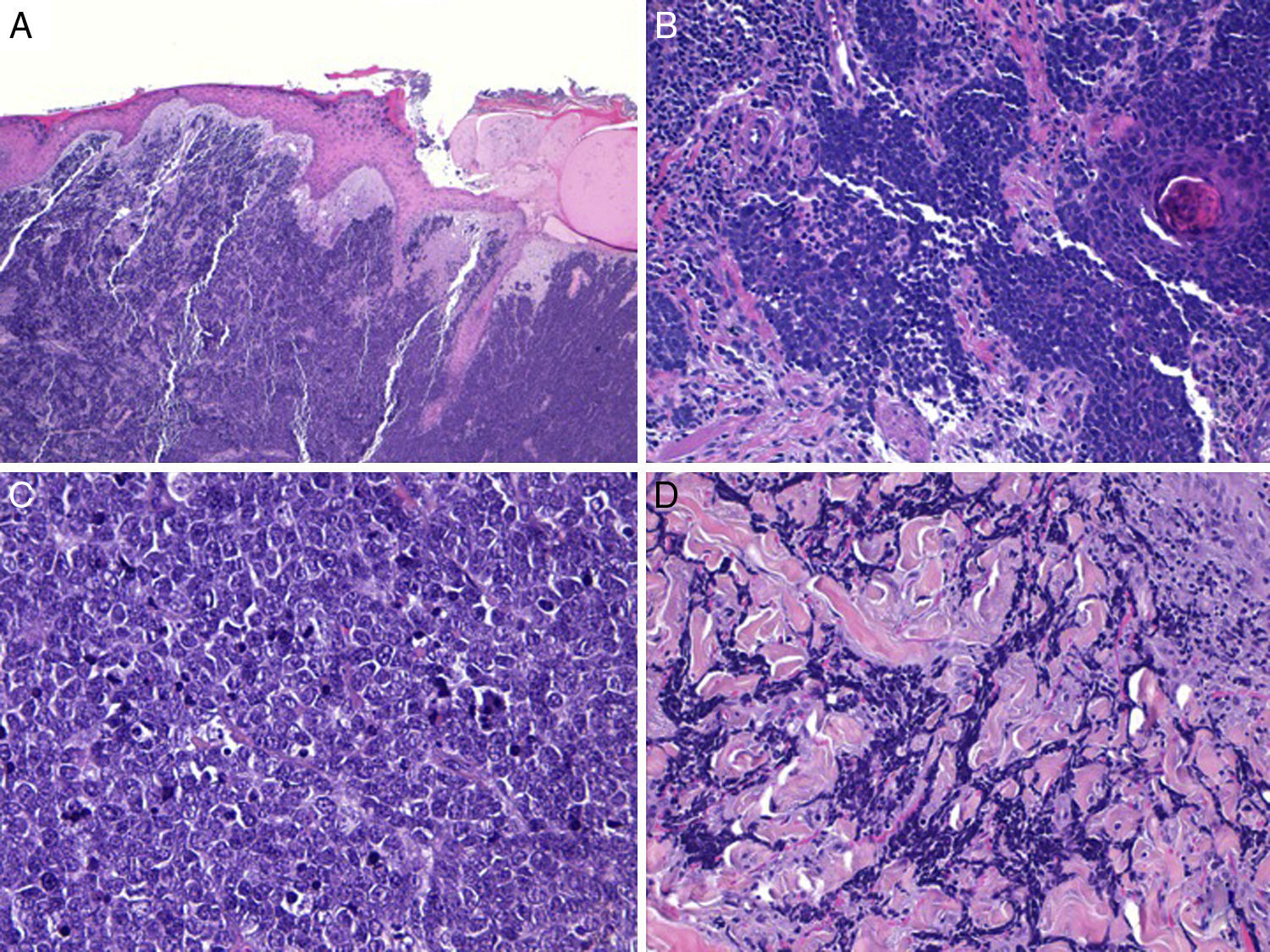
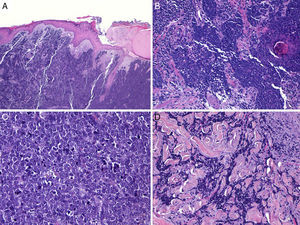
An area separating the tumor from the epidermis (Fig. 3) is usually present; epidermal ulceration is found in only about 15% of cases, and less than 10% of MCCs show epidermal involvement (epidermotropism).42,59 MCCs confined to the epidermis (MCC in situ)53,60 or MCCs with only minimal dermal involvement51 have been described. Aggregates may form in the epidermis or adopt a pagetoid pattern that can imitate other intraepidermal tumors such as melanoma as well as mycosis fungoides, Bowen disease, or Paget disease.61
Merkel cell carcinoma with an infiltrative pattern. A, Tumor in the dermis, leaving the epidermis intact; hematoxylin-eosin, original magnification ×100. B, Tumor extensively infiltrating the dermis; hematoxylin-eosin, original magnification ×200. C, Atypical cells that are small, round, and basophilic; hematoxylin-eosin, original magnification ×400. D, Infiltrate and collagen bundles inside a tumor; hematoxylin-eosin, original magnification ×200.
Trabecular, nodular, and diffuse histologic patterns of no prognostic interest have been described.62 The trabeculae reported by Toker1 in 1972 are uncommon and, when found, are usually at the periphery of the tumor. In this pattern the cells are grouped in thick trabeculae separated by a fibrous stroma, appear compact, and mimic glands or rosettes. The nodular pattern, in which the cells form solid nodules, is the most frequent. A nodular MCC generally resembles a lymphoma. The diffuse pattern is characterized by an infiltrate of small cells arranged in sheets separated by abundant stroma. Mitotic activity is high and large necrotic areas are present. In such cases the tumor resembles small cell lung cancer. It is not unusual for a tumor to exhibit more than a single pattern.
Divergent histologic patterns have been described exceptionally (in 5% of cases).63 Eccrine gland differentiation64,65 is confirmed by a finding of immunohistochemical expression of cytokeratin (CK) 7 and carcinoembryonic antigen. Cases of squamous,66 melanocytic,63 and muscular differentiation have been reported, as well as tumors with a so-called lymphoepithelioma pattern.67 A lymphoid infiltrate has been linked to tumor regression. Squamous differentiation may be fibrosarcomatous,68 leiomyosarcomatous,69 or rhabdomyosarcomatous; multinucleated giant cells may also be present.70 Cases with glandular, squamous, and melanocytic differentiation in the same tumor have been described.63 The ability of MCCs to differentiate into other cell lines suggests an origin in pluripotential stem cells71,72 rather than the traditional theory that they arise in the epidermis.
MCC presents as a collision tumor along with another lesion in 15% of cases.42 The most common adjacency is with a squamous cell tumor,53 but actinic keratosis, Bowen disease,73 and basal cell carcinoma70 have also been implicated. Other lesions that have been associated with MCC anecdotally are epidermal cysts, trichilemmal cysts, trichoblastoma, poroma,74 and lentigo maligna.
Spontaneous regression of an MCC has sometimes been reported, but the mechanism involved is unknown. Takenakaetal.75 suggested that regression might be an effect of immune system stimulation by the biopsy procedure. Histologic features in the area of regression include a perivascular lymphocytic infiltrate composed mainly of CD8+ cells and fibrosis. The host's immune defenses are thought to be determinants in the course of MCC tumors, opening the possibility of immunologic therapies in the future.
Histologic features that should be present in the pathology report to support a diagnosis of MCC are summarized in Table 1.76–78
Features Required for a Histologic Diagnosis of MCC a
| Tumor |
| Procedure type (biopsy, exeresis, re-exeresis, other:______) |
| Macroscopic tumor (present or absent) |
| Tumor location:___________ |
| Tumor size, cm:___________ |
| Tumor thickness, mm b:____________ |
| Margins (side, depth; involved structures or tumor free) (distance______mm) |
| Lymphovascular invasion (present or absent) |
| Muscle, bone, fascia, or cartilage invasion |
| Mitotic index, per mm2b:______/mm2 |
| Peritumoral lymphocytic infiltrate b (present or absent) (dense, not dense) (cell type: lymphocytic, plasmocytic, other:_____) |
| Intratumoral lymphocytic infiltrate b (present or absent) (dense, not dense) (cell type: lymphocytic, plasmocytic, other:_____) |
| Growth pattern b (nodular or infiltrative) |
| Presence of differentiation b: squamous, eccrine, melanocytic, sarcomatous, other: _______) |
| Presence of an adjacent tumor b (squamous cell carcinoma, basal cell carcinoma, actinic keratosis, other:___________) |
| Immunohistochemical analyses: |
| CK AE-1/AE-3) (positive [____%], negative) |
| CK20 (positive [____%], negative) |
| CK7 (positive [____%], negative) |
| Chromogranin (positive [____%], negative) |
| Synaptophysin (positive [____%], negative) |
| CD56 (positive [____%], negative) |
| TTF-1 (positive [____%], negative) |
| Lymph node |
| Procedure type (sentinel lymph node biopsy, lymph node dissection:______) |
| Location |
| Diagnostic hematoxylin staining: positive, negative, indeterminate |
| Diagnostic immunohistochemistry: positive, negative, indeterminate |
| Immunohistochemical technique used: ____________ |
| No. of lymph nodes examined (total):______; no. of involved nodes:_____ |
| Tumor load (percentage of area affected and size of the largest focus of metastasis ____mm) |
| Metastasis, location: subcapsular, parenchymal, germinal center |
| Extranodal spread (present, absent, indeterminate) |
| Pathological TNM stage:____________ |
Abbreviations: CK, cytokeratin; MCC, Merkel cell carcinoma; TTF-1, thyroid transcription factor 1.
Because MCCs are neuroendocrine carcinomas that express a set of CK and neuroendocrine markers, immunohistochemistry facilitates diagnosis. If optical microscopy alone is used, the risk of diagnostic error has been reported to be around 66%.79
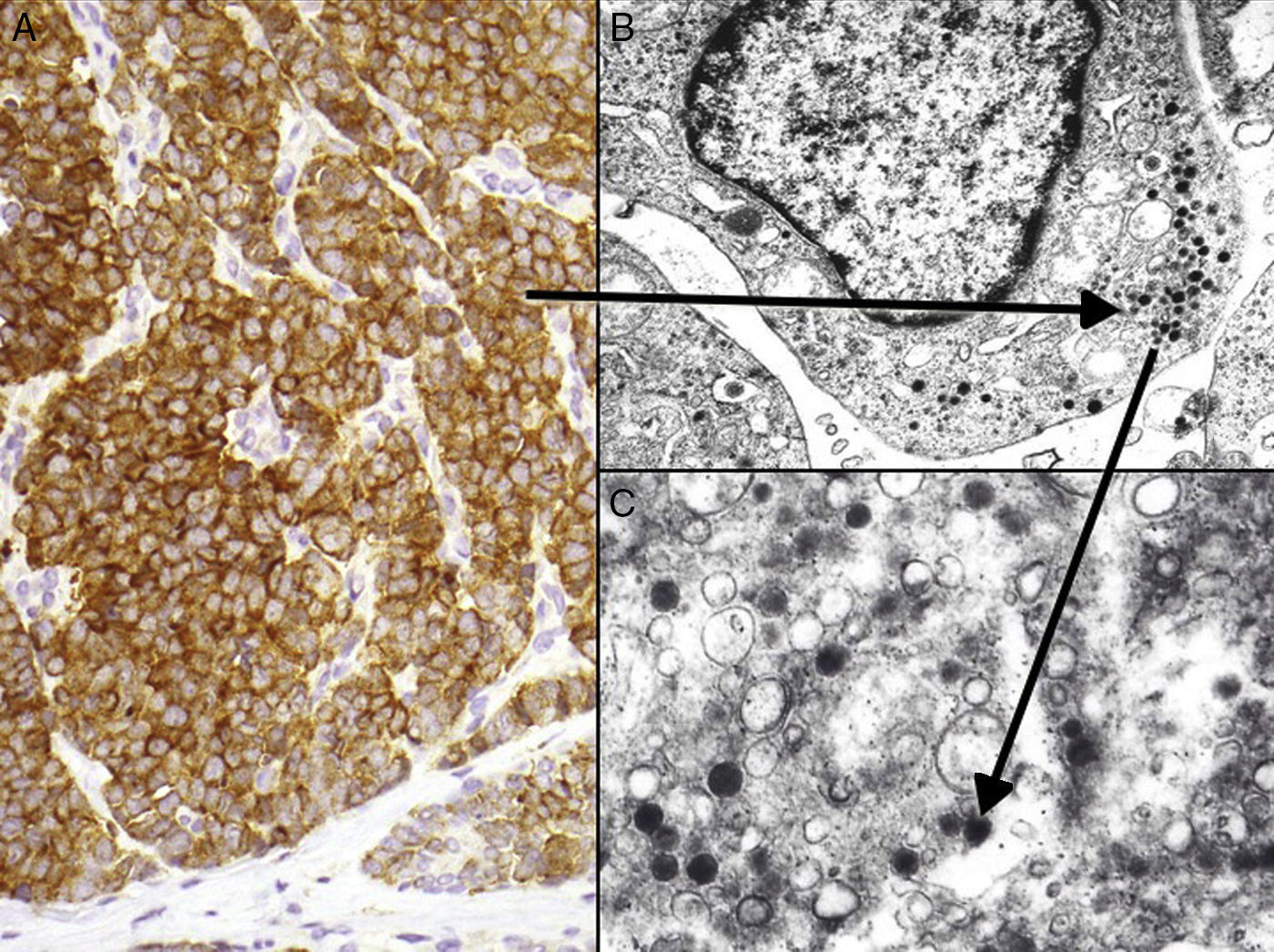
Assays show positive findings for low-molecular-weight CKs (CK8, CK18, CK19, and CK20) in most MCCs. The high–molecular-weight CK7 is not expressed. Most studies conclude that a positive result for CK20 expression along with negative results for CK7 will be highly useful, providing an accurate diagnosis in 90% of cases.80 CK20 presents a highly characteristic cytoplasmic paranuclear dot-like pattern (Fig. 4) and is considered one of the keys to diagnosis. However, the absence of CK20 expression does not rule out MCC. Cases of MCCs with CK7+ and CK20–cells have even been reported.81
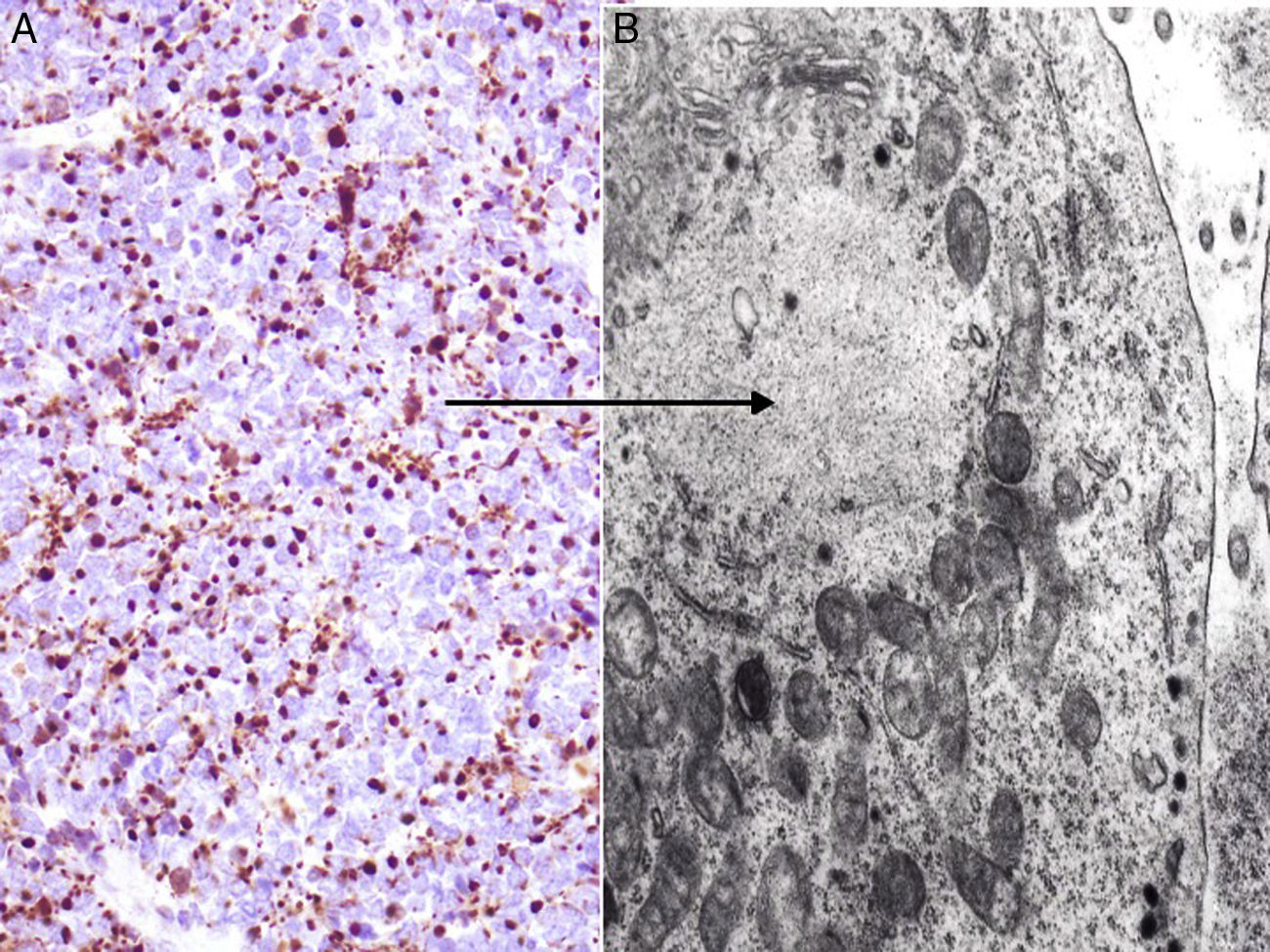
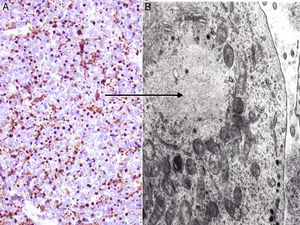
Neuron-specific enolase is a very sensitive but fairly nonspecific neuroendocrine marker that also appears in other neoplasms (melanoma, other neuroendocrine tumors, and small cell lung cancer). The proteins chromogranin and synaptophysin are present in neurosecretory granules (Fig. 5). A typical cytoplasmic staining pattern shows globules that are 100% chromogranin B, 72% chromogranin A, and 50% synaptophysin.42,82,83 Also worth mentioning is immunostaining for CD56, a glycoprotein present in neuroendocrine cells and whose expression has been described in around 94% of MCCs.84
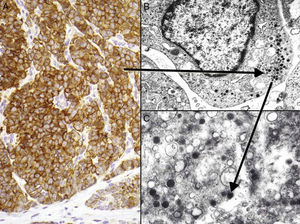
Immunohistochemistry of Merkel cell carcinoma. A, Immunohistochemical staining with chromogranin for neuroendocrine differentiation showing the characteristic granular pattern in the cytoplasm. B and C, Electron microscopic images showing electron-dense granules dispersed in the cytoplasm of cells from a Merkel cell tumor.
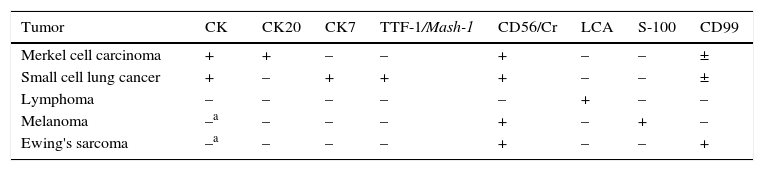
The histologic differential diagnosis must include other round and small cell tumors, mainly those that metastasize to the skin such as small cell lung cancer, poorly differentiated squamous cell carcinoma, or lymphoma or anaplastic melanoma with round cells. The principal differential diagnoses and immunohistochemical techniques applied are listed in Table 2. A definitive diagnosis of MCC requires a negative finding for the S-100 protein, the leukocyte common antigen, and high molecular weight CKs.85,86
Immunohistochemical Differential Diagnosis in MCC
| Tumor | CK | CK20 | CK7 | TTF-1/Mash-1 | CD56/Cr | LCA | S-100 | CD99 |
|---|---|---|---|---|---|---|---|---|
| Merkel cell carcinoma | + | + | – | – | + | – | – | ± |
| Small cell lung cancer | + | – | + | + | + | – | – | ± |
| Lymphoma | – | – | – | – | – | + | – | – |
| Melanoma | –a | – | – | – | + | – | + | – |
| Ewing's sarcoma | –a | – | – | – | + | – | – | + |
Abbreviations:±, MCC and small cell lung cancer express CD99 in the cytoplasm but not in the membrane; CK, cytokeratin (AE1/AE3); Cr, chromogranin; LCA, leukocyte common antigen; Mash-1, mammalian achaete-scute homolog 1 gene; MCC, Merkel cell carcinoma; TTF-1, thyroid transcription factor 1.
Immunohistochemical membrane staining for CD99 is a characteristic of Ewing sarcoma. However, between 40% and 55% of MCCs have CD99+ cells, albeit only in the cytoplasm.42,87
Thyroid transcription factor 1 (TTF-1) is a nuclear protein involved in regulating transcriptional activity during embryonic development of the thyroid gland and respiratory epithelium.88 This marker is typically positive in lung and thyroid cancers and negative in MCC, so a negative TTF-1 finding is considered more specific than a positive CK20 result, given that the latter can be negative in a considerable proportion (20%) of MCCs whereas TTF-1 is expressed in very few (3%).89–91 Recent studies have found that the mammalian achaete-scute homolog 1 (Mash-1) gene, linked to embryonic development of brain and neuroendocrine cells, is useful in distinguishing between MCC and metastasis of small cell lung cancer to the skin, especially in the rare cases when the tumor cells are TTF-1 positive. MCC does not express Mash-1, but 83% of small cell lung cancers do.91,92 Thus, a combined finding of TTF-1, Mash-1, and CK20 expression should provide greater sensitivity and specificity for distinguishing between MCC and other small cell carcinomas.
In summary, immunohistochemical detection of intermediate filaments, essentially CK2049 and neuroendocrine markers such as CD56, chromogranin or synaptophysin, and absence of expression of TTF-1, Mash-1, CK7, S-100 protein, and leukocyte common antigen offer useful information for distinguishing between MCC and other blue skin tumors with round cells (Table 2).43
Ultrastructural FeaturesElectron microscopy has played an important role in our understanding of MCC,93 but this step is currently not ordered because the combined findings of optical microscopy and immunohistochemistry provides sufficient information for diagnosis. MCC tumor cells share the characteristics of Merkel cells themselves: a lobulated nucleus and multiple small nucleoli. The cytoplasm contains abundant ribosomes and a prominent Golgi apparatus.
MCC cells have 2 characteristic ultrastructural features: intermediate filaments (Fig. 4) and electron-dense granules94 (Fig. 5). These granules in the cytoplasm typically measure between 100 and 250nm in diameter. Small cell lung cancer and other carcinomas of the neuroendocrine system also present with electron-dense granules.
Paranuclear aggregates of intermediate filaments or fibrous bodies can also be observed in the cytoplasm of neoplastic cells. Paranuclear positioning is typical of these filaments in MCC, whereas they form a perinuclear pattern in small cell lung cancer.
Cytogenetic and Molecular AnalysisCertain cytogenetic changes affect 30% to 47% of MCCs; changes are usually on chromosome 1 but have also been found on chromosomes 3, 11, and 13.95 Comparative genomic hybridization analyses have found gains and losses that are very similar to those seen in small cell lung cancer.
Studies have found alterations in the B-cell lymphoma 1 (bcl-1) oncogene96 and mutations in the tumor protein (p53) gene97 that might be related to progression and aggressiveness. The number of somatic mutations in the MCC tumor seems to be inversely related to the MCPyV load. MCPyV-positive samples show few mutations, whereas samples negative for the virus show many, especially mutations with a signature that corresponds to those observed in small cell lung cancer (i.e., the retinoblastoma protein and p53).98,99
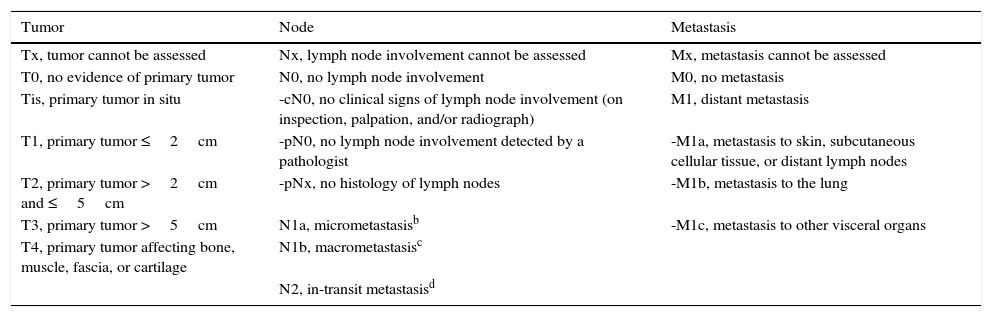
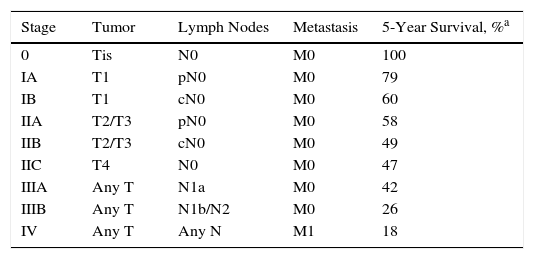
StagingStaging is of great importance for establishing prognosis and guiding proper treatment in MCC. The simple, traditional system described by Yiengpruksawan et al.41 has 3 stages: stage I, localized skin lesion (IA, <2cm; IB, ≥2cm); stage II, regional lymph node involvement; stage III, metastatic disease. The American Joint Committee on Cancer proposed a new, more complete staging system in 2010 (Table 3) based on the analysis of over 4000 cases.100 This new system highlights the importance of tumor size, regional lymph node status, and metastasis in relation to survival. Four clinical stages are possible at the moment of diagnosis (Table 4). The first two include localized disease that only affects the skin. The third includes cases with regional lymph node involvement while the fourth reflects metastatic dissemination. Five-year survival in truly localized disease (stages I and II) is about 50%, a percentage that drops to under 20% if there is metastasis (stage IV) (Table 4). Tumor size is the most important prognostic factor when the MCC is confined to the skin (stages I and II). Most studies have found that prognosis is worse when MCCs are larger than 2cm (≥T2).77 However, significant prognostic differences have not been found for tumors measuring between 2 and 5cm (T2) as opposed to tumors larger than 5cm (T3). Therefore, MCCs in both size categories are placed in stage II (Table 4). Lesions that invade the fascia, muscle, bone, or cartilage, however, are classified as stage IV (T4), indicating worse prognosis, regardless of their size.
TNM Classification of MCC According to the American Joint Committee on Cancer a
| Tumor | Node | Metastasis |
|---|---|---|
| Tx, tumor cannot be assessed | Nx, lymph node involvement cannot be assessed | Mx, metastasis cannot be assessed |
| T0, no evidence of primary tumor | N0, no lymph node involvement | M0, no metastasis |
| Tis, primary tumor in situ | -cN0, no clinical signs of lymph node involvement (on inspection, palpation, and/or radiograph) | M1, distant metastasis |
| T1, primary tumor ≤2cm | -pN0, no lymph node involvement detected by a pathologist | -M1a, metastasis to skin, subcutaneous cellular tissue, or distant lymph nodes |
| T2, primary tumor >2cm and ≤5cm | -pNx, no histology of lymph nodes | -M1b, metastasis to the lung |
| T3, primary tumor >5cm | N1a, micrometastasisb | -M1c, metastasis to other visceral organs |
| T4, primary tumor affecting bone, muscle, fascia, or cartilage | N1b, macrometastasisc | |
| N2, in-transit metastasisd |
Abbreviation: MCC, Merkel cell carcinoma.
Staging of MCC According to the American Joint Committee on Cancer a
| Stage | Tumor | Lymph Nodes | Metastasis | 5-Year Survival, %a |
|---|---|---|---|---|
| 0 | Tis | N0 | M0 | 100 |
| IA | T1 | pN0 | M0 | 79 |
| IB | T1 | cN0 | M0 | 60 |
| IIA | T2/T3 | pN0 | M0 | 58 |
| IIB | T2/T3 | cN0 | M0 | 49 |
| IIC | T4 | N0 | M0 | 47 |
| IIIA | Any T | N1a | M0 | 42 |
| IIIB | Any T | N1b/N2 | M0 | 26 |
| IV | Any T | Any N | M1 | 18 |
Abbreviation: MCC, Merkel cell carcinoma.
The new staging system considers whether the absence of lymph node involvement is confirmed by a pathologist (pN0) rather than based only on clinical or radiologic criteria. This distinction between clinical and histologic staging is very important given that up to a third of patients with apparently local disease do in fact have undetected metastasis.101 Nonetheless, micrometastasis (N1a) indicates a much better prognosis (5-year survival, 42%) than macrometastasis (N1b, 5-year survival, 26%) (Table 4).
One-year survival in patients who have metastasis at diagnosis is approximately 44%.100 Although the new staging system gives different categories for metastasis to different locations, this aspect does not affect survival.
Ethical DisclosuresProtection of human and animal subjects.The authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consent.The authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Llombart B, Requena C, Cruz J. Actualización en el carcinoma de células de Merkel: Epidemiología, etiopatogenia, clínica, diagnóstico y estadificación. Actas Dermosifiliogr. 2017;108:108–119.