A 9-year-old boy was referred to the dermatology clinic with rough punctate lesions on his eyebrows, cheeks, and both arms. His personal history was remarkable for atopic dermatitis, rhinitis, asthma, and left mesial–temporal ganglioglioma. His brain tumor was treated with surgery and local radiotherapy. Given the presence of the BRAF V600E mutation in the affected cells of the ganglioglioma, we subsequently decided to initiate the BRAF inhibitor dabrafenib at 150mg every 12hours. Three months after initiation of treatment, the patient began to develop cutaneous manifestations, for which he had not received treatment.
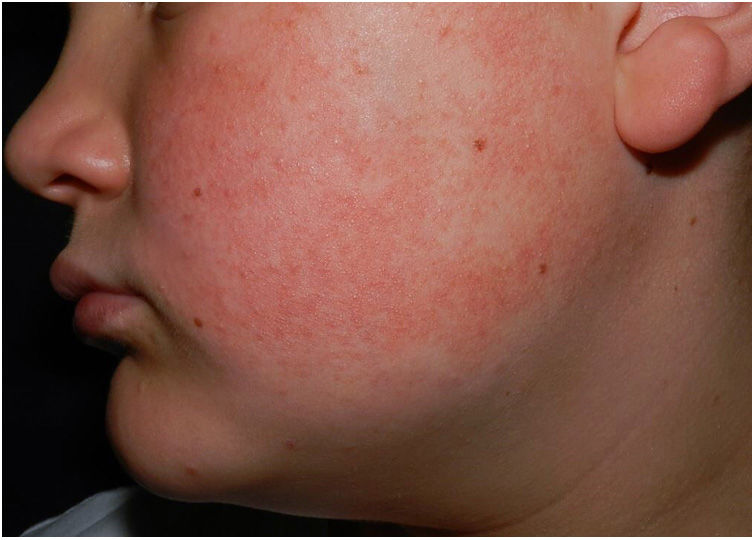
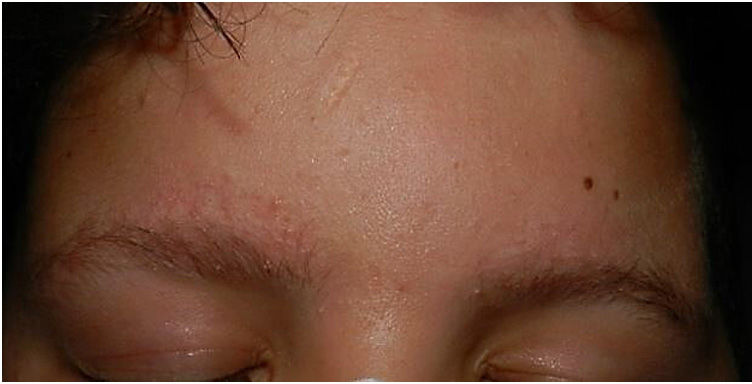
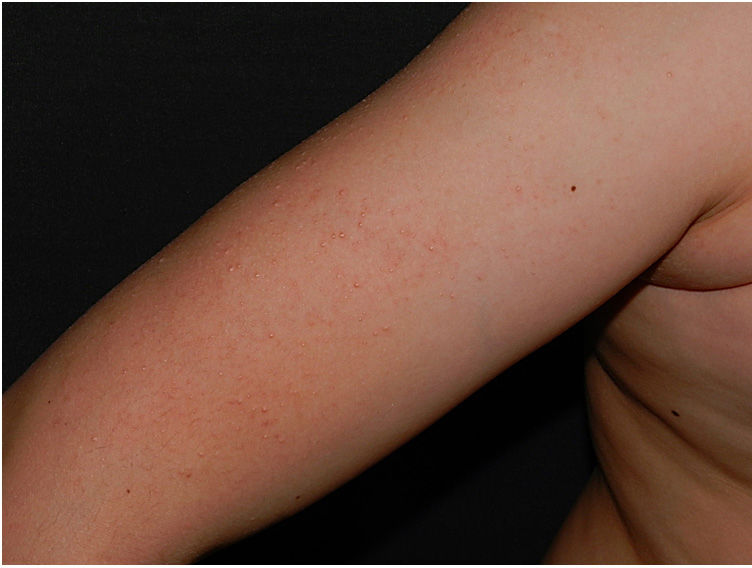
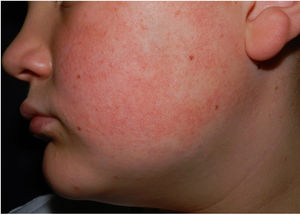
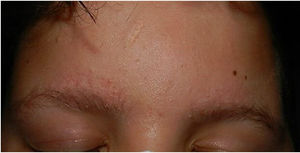
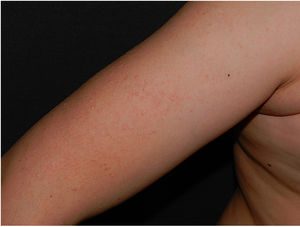
Physical examination revealed minute hyperkeratotic papules on an erythematous base on both cheeks (Fig. 1) and eyebrows (with hair loss in the middle area) (Fig. 2), as well as multiple hyperkeratotic papules on the external aspect of both arms (Fig. 3). No lesions were visible on other areas of the body or the mucosa.
Given the patient's clinical history and the cutaneous manifestations, he was diagnosed with ulerythema ophryogenes, also known as keratosis pilaris atrophicans faciei, an uncommon skin disease characterized by erythematous follicular papules that mainly affect the eyebrows, forehead, and cheeks, with occasional progression to atrophy and alopecia. The condition can appear during childhood or adulthood.1,2
The differential diagnosis is with a closely interrelated group of disorders known as keratosis pilaris atrophicans, which include atrophoderma vermiculatum and keratosis spinulosa decalvans.1,2
Ulerythema ophryogenes is considered a cutaneous marker of several congenital syndromes, including Noonan syndrome, Cornelia de Lange syndrome, Rubinstein-Taybi syndrome, partial monosomy of the short arm of chromosome 18, abnormalities of the nerves, eye diseases, and mental retardation. It is considered an autosomal dominant genodermatosis with incomplete penetrance.1–3
The V600E mutation in BRAF is common in cerebral ganglioglioma, affecting approximately 50% of patients and leading to poorer progression-free survival than gangliogliomas that do not harbor it. These mutations have generated considerable interest and have become therapeutic targets that can be treated with BRAF inhibitors, such as dabrafenib.4
BRAF inhibitors are a pioneering targeted treatment for metastatic melanoma. They were subsequently used for other indications, have a complex safety profile, and have been associated with a wide range of cutaneous adverse reactions (mainly mild, grades 1 and 2).5–7 The most common cutaneous manifestations include cutaneous exanthema, alopecia, cutaneous squamous cell carcinoma, keratoacanthoma, hyperkeratosis palmoplantaris, keratosis pilaris-type reactions, and pruritus.6,7 As for squamous cell carcinoma, it is important to note that, in contrast with adult patients, no cases have been reported with dabrafenib in pediatric patients.4
Keratosis pilaris mainly affects the extensor surfaces of the arms and the anterior aspect of the thighs and can extend to the trunk, while generally sparing the face.6 Onset of this adverse effect has been reported during the first months after initiation of dabrafenib, irrespective of the indication. According to the latest publications, it is experienced by 10%–14% of patients.4,8 Keratosis pilaris is more common in patients who receive vemurafenib (BRAF inhibitor); in contrast, incidence is lower in patients who receive combined treatment with BRAF and MEK inhibitors.7
In 2016, Khetarpal et al.9 reported the first case of keratosis pilaris atrophicans associated with nilotinib, a second-generation tyrosine kinase inhibitor, in a patient with chronic myeloid leukemia.
Our review of the literature revealed no cases of keratosis pilaris atrophicans faciei induced by dabrafenib. Here, we report the first case of ulerythema ophryogenes in a 9-year-old boy after treatment with dabrafenib for mesial–temporal ganglioglioma. Ulerythema ophryogenes is considered a marker of several congenital syndromes1,2 and does not usually present as an adverse effect of drugs. However, this was not the case in the patient we describe, and the temporal association from initiation of the drug supported the diagnosis.
This type of cutaneous manifestation is usually mild, requiring symptomatic management. Therefore, it is important to distinguish it from drug hypersensitivity reactions, which are more serious and may require medication to be interrupted.7,10 While there have been reports of spontaneous resolution on entering adolescence, treatment is usually with keratolytic agents, corticosteroids, and photoprotection, and the response is variable.10 Successful outcomes were recently reported with pulsed-dye laser treatment.3
FundingThe authors declare that no funding was received for the present study.
Conflict of InterestsThe authors declare they have no conflict of interest.