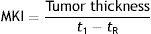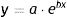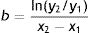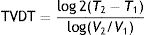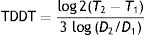Skin cancer, like other cancers, is characterized by the uncontrolled growth of transformed cells. Tumor growth has been studied for decades. We review different methods for measuring skin tumor growth and propose a new system for estimating tumor doubling time that could be useful in the management of skin cancer.
El cáncer de piel al igual que otros tumores internos está compuesto por células transformadas de crecimiento incontrolado. El crecimiento tumoral ha sido objeto de estudio desde hace décadas. En este artículo se repasan las distintas formas de medición del crecimiento tumoral cutáneo y se establecen las bases para el desarrollo de una estimación del tiempo de duplicación tumoral que pueda ser útil en el manejo de los pacientes con cáncer de piel.
Cancer is characterized by the uncontrolled growth of transformed cells. It is assumed that a tumor begins as a single transformed cell that divides, or doubles, repeatedly to form 2 and then 4 cells, and so on. Even accounting for the inevitable cell loss that occurs due to phenomena such as apoptosis and tumor necrosis resulting from poor vascularization, it has been estimated that after 40 doublings, a tumor can weigh as much as 1kg. Any subsequent doubling is potentially lethal.1 Researchers have studied tumor growth for some 80 years in an attempt to better understand the kinetics involved and improve the management of cancer. Most studies have assumed that tumor growth is exponential,1,2 but other models have been proposed.3
Studies analyzing tumor growth have largely analyzed internal tumors, generally by measuring the size of metastases using radiologic images, with the limitations that this entails.4–6 In this article, we examine the main evidence on skin tumor kinetics and review the concepts of tumor growth rate and tumor doubling time. We also propose a new method for ascertaining skin tumor doubling time.
Skin Tumor Growth RateBecause most patients or those close to them tend to remember when they first noticed a skin lesion and more or less recall its appearance, skin tumors, unlike internal tumors, offer the opportunity to assess growth kinetics. According to the National Comprehensive Cancer Network (NCCN) clinical practice guidelines on cutaneous squamous cell carcinoma (cSCC), rapid growth is a potential indicator of a high-risk tumor.7 The first obvious question, however, is what exactly is meant by rapid growth, as the NCCN does not specify any cutoff values or methods for measuring the growth of cSCCs. The situation is similar for other tumors.
To our knowledge, the first study to analyze tumor growth rate was a study published by Teloh in 1953 on basal cell carcinoma (BCC)8 in which the author defined rate of growth as follows:
Size was defined as diameter. Teloh showed that higher rates of growth (>0.7mm/mo) were correlated with increased tissue invasion.
In 1985, Fitzpatrick and Harwood reported on a series of fast-growing cSCCs they called acute epitheliomas that were associated with worse 3-year survival rates compared with cSCCs that did not exhibit rapid growth. The definition of rapid growth, however, was purely subjective, with no mention made of measurements.
In 2002, Grob et al.9 created an objective measure of melanoma growth, the melanoma kinetics index (MKI), which they expressed as follows:
where t1 was the date on which the patient or family member first noticed the visible growth of the tumor and tR the date of resection. Growth was measured in millimeters per month. The authors used Breslow thickness as an indirect measure of tumor volume, which is more difficult to calculate. They observed a proportional relationship between disease-free survival rates and different quartiles of the MKI. While they acknowledged that patient-reported times are “subjective”, they added that “subjective does not mean inaccurate or irrelevant”.9 Many authors are proponents of narrative-based medicine, which in contrast to evidence-based medicine, focuses on information that can be extracted from what patients tell their doctors during clinical interviews.10 Lin et al.,11 on analyzing sequential biopsy specimens from the same melanomas, confirmed that growth rates calculated using information from clinical histories were a reliable clinical marker.Liu et al.,12 in a 2006 Australian study investigating growth kinetics in melanoma, established that a third of melanomas could be considered fast-growing, which they defined as a growth rate of more than 0.5mm/mo. Fast-growing melanomas were thicker, had a higher mitotic rate, and occurred more frequently in symptomatic patients and patients older than 70 years. The same group subsequently identified correlations between melanoma growth rate and proliferation markers Ki-67 and phosphohistone H3.13 This characterization of fast-growing melanomas was corroborated by Martorell-Calatayud et al.,14 who, on observing fewer associations with a history of sunburn, suggested the occurrence of another pathogenic pathway.
In 2010, Tejera-Vaquerizo et al.,15 followed by Nagore et al.,16 showed that growth rate was an independent prognostic factor in melanoma. Growth rate has also been directly linked to a greater likelihood of sentinel node positivity in this setting.17
A correlation has also been found between melanoma growth rate and metastasis occurrence, with faster-growing tumors metastasizing earlier.18 One study identified a critical time of metastasis occurrence after which fatal events may be seen and, paradoxically, faster-growing melanomas begin to metastasize at a greater thickness than thin melanomas.19
Although BRAF mutations induce cell proliferation through interference with the MAP kinase pathway, they are not necessarily associated with faster-growing melanomas, although they are linked to higher mortality.20 Co-occurrence of BRAF and TERT promoter mutations, has, by contrast, been associated with fast-growing melanomas,21 which, more recently, have also been linked to fibroblast growth factor receptor 2 mutations, a potential marker of rapid growth.22
Evidence on growth rates in nonmelanoma skin tumors is more limited. In the case of cSCC, Cañueto et al.23 calculated growth rate as the ratio between tumor diameter in mm and progression time. Using partition regression techniques, they determined that a rate of 4mm/mo was the optimal cutoff for identifying fast-growing cSCCs. They also found that this cutoff was associated with a greater tendency for and earlier occurrence of regional lymphatic recurrences. Similarly to with other tumors, growth rate calculations for cSCCs are limited by subjective factors. Nonetheless, while BCCs grow consistently over time, this is less evident in the case of cSCCs,24 whose size appears to depend more on growth rate than on time untreated. Time bias is lower in fast-growing tumors. In other words, these tumors tend to be diagnosed sooner because they are noticed earlier. In addition, growth from diagnosis to definitive treatment is easily measurable and could be useful for reliable calculations. The inclusion of depth measurements obtained by imaging techniques such as ultrasound could enable the calculation of tumor volume and add another element of precision to growth assessments.
Analyses of BCC growth rates include the early study by Teloh8 mentioned above and a descriptive study by Betti et al.,25 who used both depth (Breslow thickness) and lateral extensions of excised tumors to assess rate of growth. The authors suggested that depth was a more useful measure for nodular subtypes, while lateral extension was more useful for superficial subtypes. In a study conducted in New Zealand, Sykes et al.26 investigated the growth rate of BCCs using axial and longitudinal measurements and area growth rates in mm2/mo, and confirmed that BCC is a slow-growing tumor and that prognosis is not significantly affected by diagnostic delays.
Tumor Doubling Time As A More SuitablemeasureThe evidence to date on the kinetics of cutaneous tumors is based on growth rates measured in millimeters per month. As explained, calculations are based on tumor thickness or diameter as a surrogate for the more desirable measurement of tumor volume.
Diameter could also be used to estimate tumor growth based on an exponential growth model designed by Tejera-Vaquerizo et al.27 to estimate the effects of diagnostic delays due to the COVID-19 lockdown on SCC and melanoma growth. In the model, the dependent variable (y=lesion size) and independent variable (x=time) are linked as follows:
Both a and b are adjustable, with a representing the initial size of the tumor and b its growth (the higher b is, the faster the tumor will grow). To estimate exponential tumor growth, it is necessary to know y (size) and x (time) at 2 distinct moments. In the equation, y1 is the size of the tumor when it was first noticed by the patient or a family member and y2 is its size at the time of surgery. Likewise, x1 and x2 indicate, respectively, when the tumor was first noticed and when it was excised.
This equation can be used to calculate tumor growth when diameter size is known at 2 moments in time. Tumor doubling time, however, would be a more reliable indicator of kinetics.
Schwartz,2 in an evaluation of a potential exponential tumor growth model, used tumor volume measurements taken at 2 moments in time, thus calculating tumor volume doubling time (TVDT) as follows:
where T is the time and V is the volume at 2 different moments.It would be simpler, however, and probably more practical in the case of skin tumors, to calculate tumor diameter doubling time (TDDT), which would be as follows2:
where T is the time and D is the diameter at 2 different moments.We present 2 examples of this calculation applied to SCCs on the scalp:
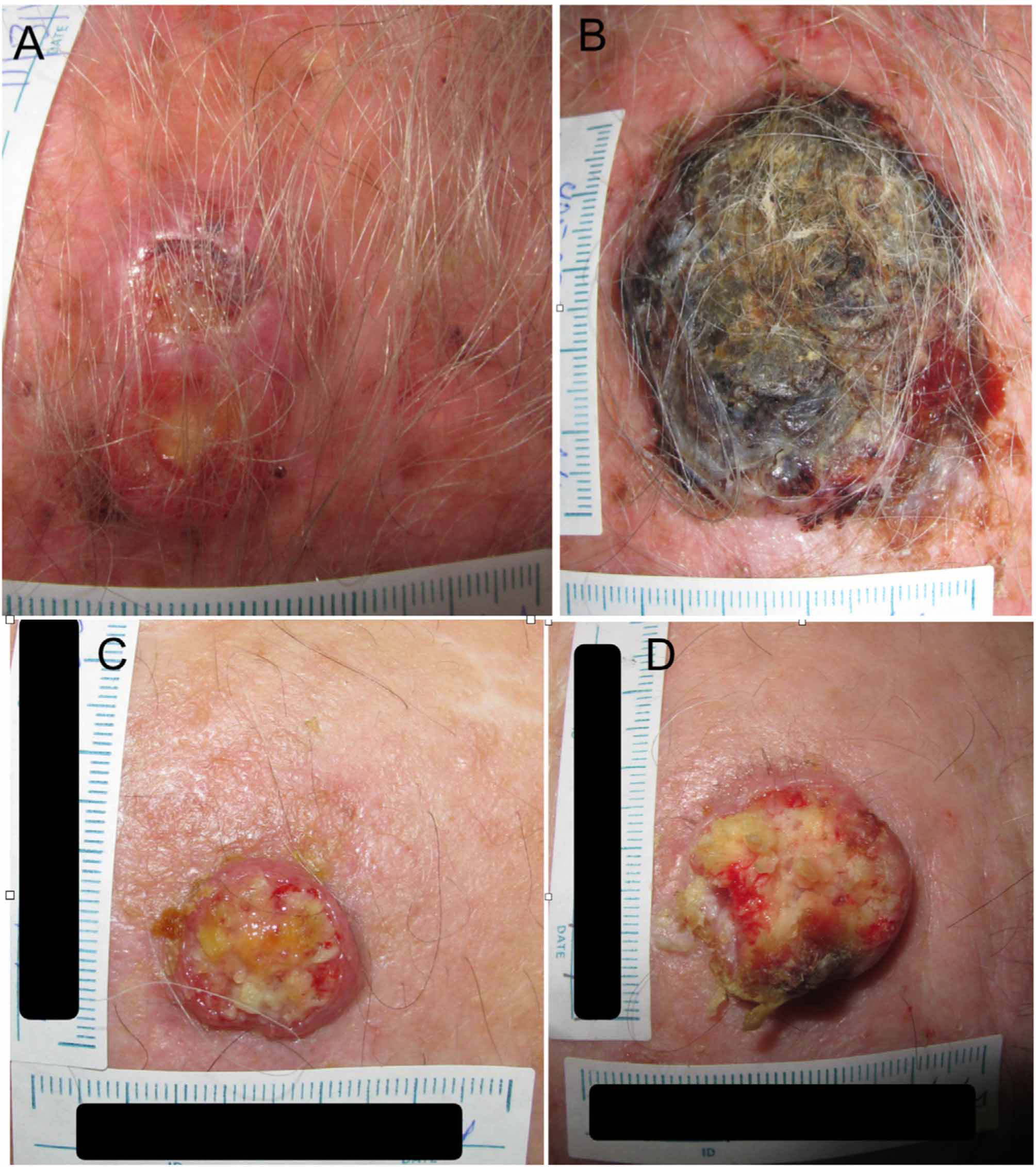
Example 1 (Fig. 1A and B) involves an SCC located on the scalp with a diameter of 27mm at T1 (D1) and 53mm at T2 (D2) and a time from T1 to T2 (T2−T1) of 84 days. The corresponding TDDT would be 7.3 days.
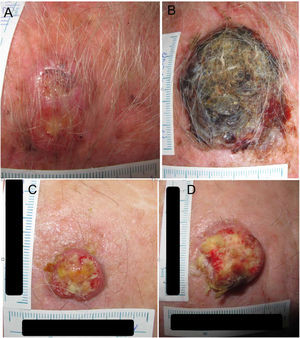
Example 2 (Fig. 1C and D) involves another SCC located on the scalp with a D1 of 20mm, a D2 of 28mm, and a T2−T1 of 133 days. In this case, the TDDT would be 16.1 days.
A shorter TDDT in lesions with a similar initial size would ultimately indicate a higher-risk CSS, even with a shorter time to diagnosis.
TDDT, however, is not practical in routine clinical practice, as in the vast majority of cases, the only known diameter is that measured at the time of the visit.
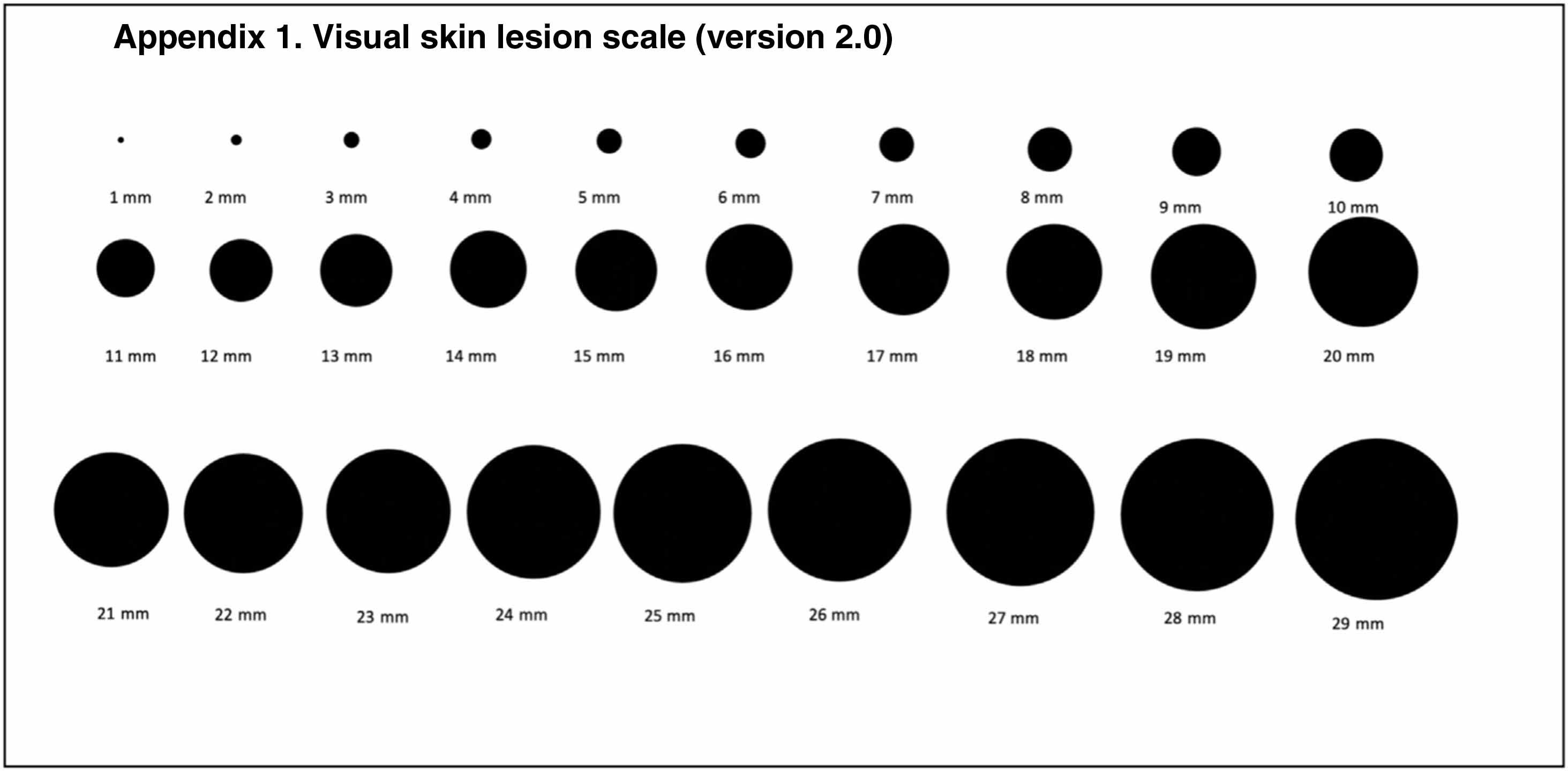
To overcome this limitation, we propose using a visual scale consisting of circles ranging from 1mm to 29mm (Fig. 2) (Supplementary Table 1) to estimate D1. Just as a patient can more or less recall when they first noticed a lesion, they may also recall its approximate size. We believe that this estimate is subject to the same uncertainty as that described for time of onset.
We are currently investigating the potential applicability of estimated TDDT (eTDDT) in the field of skin cancer. During the COVID-19 pandemic, we conducted a multicenter study on the impact of lockdown on melanoma and cSCC size and thickness in Spain.28 The researchers were asked to show patients the visual scale in Fig. 2 at the first visit to estimate the size of the tumor when they had first noticed it. More than 90% of patients provided an estimate (unpublished data). In the coming months we will have sufficient data to attempt to characterize and link eTDDT to different clinical and pathologic features of the tumors analyzed.
Just as Liu et al.12 in their characterization of tumor growth rate determined that up to a third of melanomas were fast growing, it might be possible to apply eTDDT percentiles to determine, the proportions of SCCs, melanomas, and other types of skin cancer that are fast or slow growing. Such estimates could inform both treatment and follow-up decisions.
In our opinion, eTDDT is a simple, novel tool that is worth analyzing.
Conflicts Of InterestThe authors declare that they have no conflicts of interest.