Basosquamous carcinoma (BSC) is an uncommon variant of basal cell carcinoma (BCC), characterized by areas of basaloid differentiation and areas of epithelioid (squamous) differentiation, and is associated with greater local aggressiveness and metastatic potential. When surgical excision and radiotherapy are not therapeutic options, Hedgehog pathway inhibitors (HHIs) may represent an alternative treatment.
A 40-year-old man with a past medical history of hepatitis C virus infection and HIV infection (stage B2) on darunavir/cobicistat/emtricitabine/tenofovir was being monitored for a large superficial BCC on the right pectoral region, which required multiple sessions of photodynamic therapy and topical imiquimod. Radiotherapy was declined because of the patient's young age.
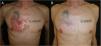
The patient was lost to follow-up and returned 2 years later with a lesion consisting of a 13×10-cm infiltrative plaque and a 4×4cm central ulcer (Fig. 1A). Surgical excision of the central ulcer was performed. Histology revealed areas consistent with BCC differentiation, positive for Ber-Ep4, together with areas of squamous differentiation with negative immunohistochemistry, leading to a diagnosis of BSC (Figs. 2 and 3).
Histopathology of the central ulcer. (A) Hematoxylin–eosin, 40×: squamous differentiation, predominant in central ulcer sections. (B) Hematoxylin–eosin, 40×: basaloid differentiation with peripheral palisading and clefting near the resection margins. (C) Ber-Ep4, 4×: strong positivity in BCC areas and negativity in regions of squamous differentiation. (D) Ber-Ep4, 4×: section from an ulcer margin showing Ber-Ep4–positive basaloid cells and Ber-Ep4-negative squamous cells.
Afterwards, sonidegib 200mg every 48h (per product label) was initiated concomitantly with ongoing HIV therapy. The patient tolerated adverse effects well (grade 1 creatine kinase elevation, muscle cramps, mild weight loss, and alopecia) and exhibited a favorable clinical response, with complete tumor involution at 8 months, allowing dose spacing to every 72h and later to twice weekly (Fig. 1B). At this latter dose, the patient developed local recurrence at one tumor margin, prompting a return to dosing every 48h. The lesion remained stable at the last follow-up 18 into therapy.
BSC is rare, representing 4.8% of cutaneous tumors,1 and histologically consists of a basaloid neoplastic proliferation with foci of squamous carcinoma. It occurs predominantly in men, with a mean age of 70 years, and most commonly on the head.1 It may behave aggressively, with frequent recurrences and an increased propensity for metastasis.
There are no specific therapeutic recommendations for this tumor type. As with BCC, surgical excision is the first-line therapy. When tumors are considered difficult to treat2 and radiotherapy is contraindicated, HHIs (sonidegib and vismodegib) may be an alternative.
Publications on the use of HHIs in BSC are scarce. This may be related to early reports suggesting an increased risk of squamous cell carcinoma (SCC) with vismodegib,3 although subsequent studies have shown that this risk is comparable to that of control populations.4,5 No published evidence suggests an increased risk of SCC with sonidegib.6
Sahuquillo-Torralba et al. reported a 45-year-old woman with an aggressive frontal BSC infiltrating the meningeal membranes who achieved complete remission 6 months into vismodegib.7 Regarding sonidegib, only 2 cases have been described6: a 59-year-old woman and an 89-year-old woman, both with large ulcerated BSCs, who achieved partial responses at 3 and 4 months with up to a 50% reduction in tumor size.
Our patient also had HIV infection. People with HIV infection have higher risk of cutaneous malignancies, including melanoma and nonmelanoma skin cancer. Specifically, the risk of BCC and SCC increases 2- and 5-fold, respectively, and is highest when HIV infection is poorly controlled (CD4 <200cells/mL or viral load >10,000copies/mL).8
Sonidegib is metabolized hepatically via cytochrome CYP3A4. Darunavir and cobicistat are potent CYP3A4 inhibitors; therefore, concomitant use increases sonidegib plasma levels. Very few reports describe HHI therapy in BCC patients on antiretrovirals, and none in BSC. Hoffmann et al described a 51-year-old patient with >100 unresectable BCCs on antiretroviral therapy who achieved complete remission 9 months into sonidegib 200mg every 2 days.9 Similarly, Fania et al reported a difficult-to-treat nasal BCC with partial response to alternate-day sonidegib.10
In conclusion, we report a case of HIV infection and difficult-to-treat BSC with good clinical response to sonidegib. HHIs may represent a therapeutic alternative, with appropriate dose adjustment to account for potential interactions with antiretroviral drugs.
Conflicts of interestThe authors declare no conflicts of interest.




 Artículo disponible en español
Artículo disponible en español