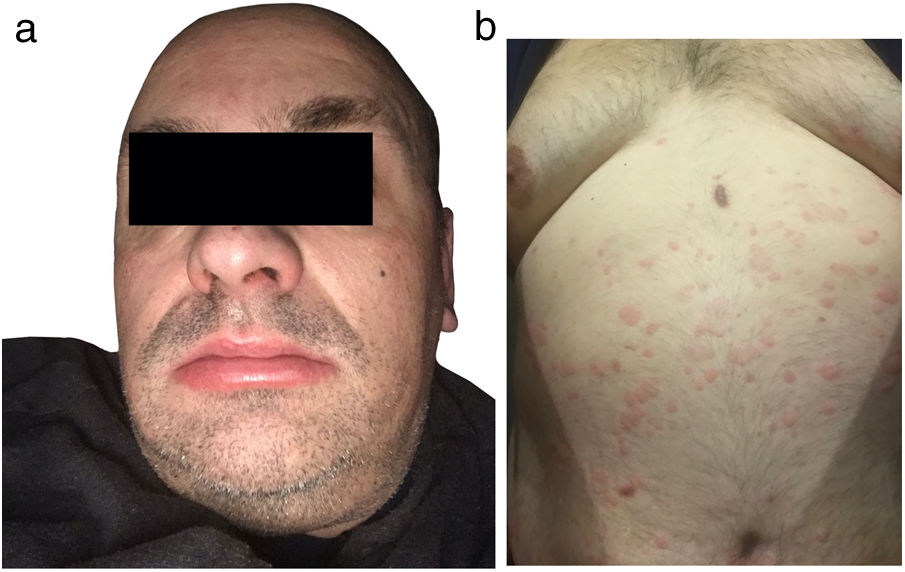
A 49-year-old male, with nasal polyps since youth, experienced a 15-month outbreak of urticaria at the age of 40, characterized by multiple pruritic wheals scattered over the trunk, which lasted <24h, accompanied by lip and eyelid swelling, and dysphonia. No fever or arthralgias were described by the patient who received 10mg/8h rupatadine, replaced by 20mg/8h ebastine, and then by 20mg/6h bilastine until the wheals disappeared.
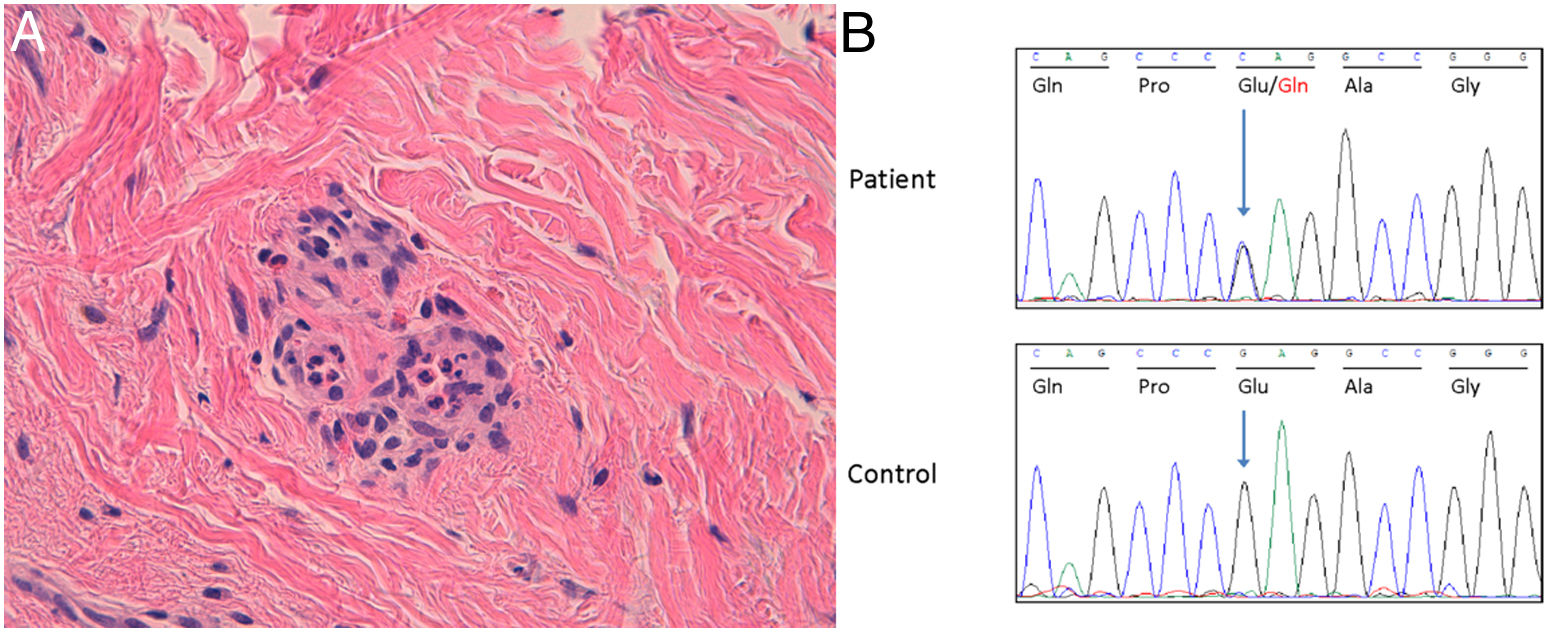
Currently, the patient presents to the clinic with a 5-month history of generalized pruritic wheals (Fig. 1) of similar characteristics treated with 10mg/6h cetirizine. However, unsatisfactory control of the condition has been observed, which has persisted for up to 6 months. The patient reported episodes of pain in the upper third of his right arm. The biopsy of a wheal (Fig. 2A) revealed the presence of a slight edema with dilated small vessels in the upper dermis, perivascular inflammatory infiltrate composed of neutrophils and occasional eosinophils, without fibrinoid necrosis in vessels, hemorrhage, or leukocytoclasia, compatible with urticaria.
(A) Hematoxylin–eosin ×200. It is confirmed that the infiltrate is composed of neutrophils and occasional eosinophils. No necrosis, or hemorrhage, or leukocytoclasia are observed. Morphological pattern is consistent with urticaria. (B) Sanger chromatogram of the index patient (top panel) and a healthy subject (bottom panel). The arrows indicate the position of the nucleotide where the MEFV variant is located. The Reference Sequence (RefSeq, https://www.ensembl.org/index.html) for the MEFV gene is NM_000243.2. Ala, alanine; Gln, glutamine; Glu, glutamic acid; Gly, glycine; Pro, proline.
Lab test results revealed leukocytosis of 17.12×10×9/L (normal: 4800–10800), neutrophilia of 10750 (normal: 1400–6500), C-reactive protein of 11.9mg/L (normal: 0.2–5.0), and interleukin 6 (IL-6) of 16.9pg/mL (normal: 5.3–7.5). Immunoglobulin E (IgE) was 106IU/mL (normal: 0–150). Serum immunofixation showed a monoclonal component of immunoglobulin G (IgG)-kappa type, without monoclonal component in urine. Alpha-1-antitrypsin, ceruloplasmin, antinuclear antibodies, rheumatoid factor, liver and kidney microsomal antibodies (LKM), antimitochondrial antibodies, anti-smooth muscle antibodies, anticardiolipin antibodies, and antineutrophil cytoplasmic antibodies were within normal parameters. A thoracoabdominal CT scan revealed the presence an osteolytic lesion in the diaphysis of the right humerus, suggestive of enchondroma. No lymphadenopathy was observed.
Genetic screening for autoinflammatory diseases was performed using a Next-Generation Sequencing panel with coverage>10×. No germline or somatic variants were detected in the studied genes classifiable as pathogenic or likely pathogenic based on the criteria of the American College of Clinical Genetics and Genomics. A heterozygous variant p.(Glu148Gln) was identified in exon 2 of the MEFV gene, which was classified as a variant of uncertain significance (Fig. 2B). Anakinra was not administered due to resolution of the wheals.
A diagnosis of Schnitzler syndrome (SS) was achieved based on the Strasbourg criteria,1 showing chronic urticaria and monoclonal gammopathy of IgG-kappa type as major criteria, and osteolytic lesion in the right humerus, neutrophilic infiltrate in skin biopsy, and neutrophilia as minor criteria. SS2,3 presents with self-limiting, long-lasting urticaria flares, poorly controlled with antihistamines. Years can pass between flares. Skin biopsy in SS shows vasculitis, neutrophil infiltration, edema, and leukocytoclasia. In this case, the biopsy showed a perivascular inflammatory infiltrate composed of neutrophils, without vasculitis. In the review by Koning et al.,4 only 20% of patients showed vasculitis on the biopsy.
Urticaria can be associated with IL-6, as it can activate and degranulate mast cells, which in turn release IL-6 and other inflammatory mediators, including histamine, leukotrienes, and prostaglandins, causing vasodilation and extravasation of cutaneous and subcutaneous plasma. This patient had elevated IL-6. Osteomuscular pain is a common finding in patients with SS. Radiologically, increased bone density, lytic bone lesions,5 and periosteal thickening may appear. Pain in the upper third of the right arm was attributed to the osteolytic lesion of the humeral diaphysis.
The test confirmed the monoclonal component of IgG-kappa type. Although the classic form of the monoclonal component is immunoglobulin M (IgM), there is another less frequent variant form of SS, in which the monoclonal component is IgG type, as in this case. Life is not shortened in SS individuals, but 12% of patients may progress into lymphoproliferative processes, with the most frequent being Waldenström's macroglobulinemia.3
Postzygotic mutations of the NLRP3 gene have been reported in patients diagnosed with the SS-variant.4 The genetic test performed did not demonstrate either germline or postzygotic mutations in this gene, or in any other, except in MEFV. In this gene, the heterozygous variant p.(Glu148Gln) was identified, which was associated with familial Mediterranean fever6,7 (FMF) in patients with fever, arthritis, abdominal pain, and urticaria. The MEFV gene encodes pyrin, a protein that inhibits the inflammasome. A few cases have been published on the presence of chronic urticaria associated with mutations in MEFV.8–10 In FMF, elevated IL-6 is observed attributed to inflammasome hyperactivity, due to changes to the pyrin function, normalizing in intercrisis periods, which may be related to urticaria.
Nasal polyposis has been associated with autoinflammatory disease. In this case, it is difficult to attribute a causal relationship between the heterozygous variant p.(Glu148Gln) in the MEFV gene and SS.
FundingNone declared.
Conflicts of interestNone declared.