The incidence of sexually transmitted diseases has been on the rise in our setting for decades. These infections represent not only an individual problem, but also a problem of public health. Therefore, the management of STDs involves reducing community incidence, which means that common issues in the clinical practice such as failure to attend may become a more complex problem, which adds to the difficult and delicate task of locating sexual contacts that would benefit from screening and the appropriate treatment. On the other hand, STDs have direct legal implications in cases of underage patients, or suspected sexual assault.
Therefore, the correct handling of these scenarios requires knowledge of the legal framework that regulates them. Dermatologists are clinically trained and prepared to deal with these conditions. Nonetheless, the legal issues involved are often difficult to solve.
This document stands as a simple reference guide to help solve the main legal issues we may encounter in a consultation when dealing with STDs.
La incidencia de las infecciones de transmisión sexual está aumentando en nuestro medio desde hace décadas. Estas infecciones representan un problema no solo individual, del paciente que las presenta, también de salud pública y, por tanto, su manejo implica el objetivo de reducir su incidencia en la comunidad. En este sentido, cuestiones habituales en la consulta, como son las incomparecencias, pueden suponer un problema más complejo; a lo que se añade la difícil y delicada tarea de localizar contactos sexuales que merecerían un cribado o un tratamiento adecuado. Por otro lado, se trata de un grupo de enfermedades con implicaciones legales directas cuando se trata de pacientes menores o si son secundarias a una agresión sexual.
El manejo correcto de las infecciones de transmisión sexual requiere, por tanto, de un conocimiento del marco legal que las ampara. Los dermatólogos estamos clínicamente formados y preparados para atender estas enfermedades; sin embargo, a menudo los aspectos legales que conllevan resultan difíciles de resolver.
Este documento pretende ser una guía sencilla, de consulta, para ayudar a resolver los principales temas legales que podemos encontrarnos en una consulta de infecciones de transmisión sexual.
In recent decades, we have witnessed an increase in the incidence and prevalence of sexually transmitted infections (STIs). The management of these diseases has public health implications, as our goal, in addition to controlling them on an individual level, is to protect against their spread among the population. On the other hand, their management has very clear legal implications in some cases, especially when patients are minors or if these STIs have appeared in the context of sexual assault.
We, as specialists in Dermatology and Venereology, are trained in the prevention, diagnosis, and treatment of STIs; however, proper care also requires knowledge of the legal framework that supports them, and we often find ourselves very limited in accessing sources that compile and provide information on this subject.
This document aims to provide practical information to help solve the main medical-legal conflicts we may encounter when managing STIs, answering some of the most frequently asked questions in the clinic: how to conduct a proper contact study, how to communicate results remotely, how to manage an STI in underage patients, and how to proceed regarding the management of a victim of sexual assault.
To this end, members of the STI and HIV Research Working Group of the Spanish Academy of Dermatology and Venereology (AEDV) (RP, PG, IF, AC), along with an expert in legal medicine (PG), conducted a search across current legislation on the subject using the main digital information search engines, the organizations official websites, and consensus documents from various Spanish medical societies. Additionally, a bibliographic search was conducted in English on PubMed and the Cochrane Library using the terms “STIs,” “legal aspects,” “contact tracing,” “absenteeism,” “sexual assault,” and the latest guidelines on STIs from the U.S. Centers for Disease Control and Prevention (CDC), the International Union Against Sexually Transmitted Infections (IUSTI), and the British Association for Sexual Health and HIV (BASHH) were reviewed.
Contact tracingContact tracing is the process by which the sexual partners of a patient diagnosed with an STI are identified and informed of their exposure, offering them care and treatment by a health care professional. This has clinical and public health benefits for 3 main reasons1:
- 1.
For the patient (index case): reducing reinfection rates.
- 2.
For partners (contacts): preventing potentially serious infections or diseases and treating curable infections.
- 3.
For the community: breaking the transmission chain at population level.
There are 3 basic modalities for contact tracing: notification by the index case (patient referral [PaR]), notification by the health care professional (provider referral [PrR]), and conditional notification (conditional-patient referral [CpR]), through which the patient is initially responsible for notification, but if they fail to do so within a specific timeframe, the health care professional takes over. Another additional tool used in some countries is the direct provision of drug to contacts by the index case (patient-delivered partner therapy [PDPT] or expedited partner therapy [EPT]2,3). Fig. 1 illustrates these 4 methods, including their advantages and disadvantages.
PaR can be reinforced by providing the patient with written information about the infection to give to their contacts, as it has been shown that the provision of educational materials increases partner treatment success.4,5 Additionally, the use of new technologies—text messages, emails, mobile apps, automated websites—allows for anonymous communication between the patient and the partner, which can speed up the notification process.4 A systematic review concluded that, although studies found high levels of interest and acceptability for electronic notification, there was little evidence of actual use.6
EPT is a process by which the contact receives treatment without being assessed by a health care professional, either through direct medication administration or by providing medical prescriptions.7 This modality is not currently considered in Spanish legislation; however, it is supported by clinical studies for the treatment of gonorrhea and chlamydia in heterosexual couples and could play a role in trichomoniasis. It is not recommended for syphilis or in men who have sex with men due to the high risk of coexisting STIs.
A Cochrane review failed to identify a single optimal strategy for contact tracing for any particular STI,4 so it should be individualized according to each clinical scenario. According to a systematic review, between 58% and 93% of patients prefer to communicate the infection themselves (PaR). However, only 20% up to 30% of partners ultimately came in for evaluation.8
Contact tracing periodIt is often difficult to pinpoint the exact moment of infection for the index case. Contacts should be traced over time depending on the type of infection, based on the natural history of each STI, the patient's history, and laboratory tests. Table 1 shows the recommended contact tracing periods for each infection.9
Recommended period for sexual contact tracing and notification.
| Infection (in alphabetical order) | Recommended periods |
|---|---|
| Chancroid | 10 days |
| Chlamydia trachomatis (including LGV) | 6 months |
| Donovanosis | Up to 1 year depending on estimated time of infection |
| Epididymo-orchitis | 6 months |
| Gonorrhea | 3 months |
| Hepatitis A | According to estimated time of infection or 2 weeks before jaundice |
| Hepatitis B | According to estimated time of infection or 2 weeks before jaundice |
| Hepatitis C | Up to the estimated time of infection if the index case or contact is HIV positive (only men who have sex with men) |
| HIV | 3 months in recent infection or from the last negative HIV test, or guided by sexual history if no tests have been conducted |
| Non-gonococcal urethritis | 4 weeks |
| Pelvic inflammatory disease | 6 months |
| Pubic lice | 3 months |
| Scabies | 2 months |
| Syphilis | - Primary: 3 months prior to clinical onset |
| - Secondary: 6 months | |
| - Early latent syphilis: 2 years | |
| - Late latent and tertiary syphilis: >30 years | |
| Trichomonas vaginalis | 2 months |
| Anogenital warts (HPV) | Contact tracing is ill-advised. Sexual partners may be invited for informational purposes |
| Herpes simplex virus | Contact tracing is ill-advised. Asymptomatic partners may be tested with specific serology to provide information on transmission risks |
HIV: human immunodeficiency virus; HPV: human papillomavirus.
Contacts should undergo appropriate testing to detect the infections to which they have been exposed and be treated based on the results. In addition, depending on their history and risk behaviors, the contact should be screened for other STIs (Grade A, Level I).9
Epidemiological management, in which treatment is administered before laboratory confirmation of infection, has a lower level of recommendation (Grade C, Level IV)9 and should generally be avoided except in the case of syphilis.10
The treatment regimen should be the same as that recommended for the index case, except in pregnant patients or known allergies. The use of condoms should be recommended until the treatment is complete and symptoms have disappeared.7
Ethical–legal issuesOne of the main factors associated with poor control of STIs is the ethical–legal difficulties that arise during contact tracing. In Spain, there is no legislation that specifically addresses the special complexity of STIs. According to the national legal framework, the following questions arise:
- 1.
Is there a legal obligation to notify? Who is responsible for notifying contacts? Yes. From a legal and ethical standpoint, the primary responsibility for contact tracing lies with the index case (patient referral), who has the legal obligation to inform their contacts of the risk of having contracted a serious disease.11–13 Failure to do so could result in a criminal offense of intentional injury.14
- 2.
What if the index case refuses to inform? The physician must document this in the health record and warn the patient that if they fail to fulfill their duty to inform, the health care professional may be exempt from the duty of confidentiality due to the serious risk to third parties.11 Contact tracing by the health care professional (provider referral and contract referral) presents significant legal and ethical challenges regarding data confidentiality, professional secrecy, and the duty to assist.12,15–17 To safeguard other important interests, such as the physical integrity of third parties, the ethical and legal framework would allow the health care professional to breach professional secrecy, access confidential personal data, and notify contacts.13,17–20
- 3.
What if the patient does not know or refuses to provide the contacts’ information? The General Health Act provides for citizens’ duty to cooperate, including abstaining from actions that hinder or prevent public health interventions.21 In such situations, we can only inform the patient of their obligations, the consequences of non-compliance, and document everything in the health record.
Despite all of the above, in doubtful cases or when balancing the rights involved is particularly complex, the Ethics Committee of the relevant hospital or the hospital legal services may always be consulted to provide support in clinical decision-making.
Remote communication and absenteeism in specialized STI consultationsAccording to the WHO, telemedicine involves “the delivery of health care services remotely […] using information and communication technologies for the exchange of valid information for the diagnosis, treatment, and prevention of diseases […], with the aim of improving the health of individuals and their communities”.22 This form of health care offers advantages not only in terms of accessibility but also in efficiency, significantly reducing costs (travel, lost work hours, etc.).23
Although its use has been prioritized for patients with chronic conditions, some studies indicate that in the context of STIs and HIV, it can facilitate management and interrupt transmission in the population.24 It has already been used to improve prevention messaging, increase acceptance of pre-exposure prophylaxis (PrEP) for HIV, optimize clinical intervention, and assist mental health services.25 However, in this field, telemedicine presents privacy challenges vs other areas of care. The legal implications concerning security, privacy, confidentiality, data protection, and medical liability are significant.26 In the absence of a specific regulatory framework, existing European and national laws or directives regarding patient autonomy, professional secrecy, data protection, privacy, and confidentiality (Annex 2.1)27 will be applied subsidiarily.
The basic principles of telemedicine are outlined in Table 2.28
Basic principles of telemedicine.
| Mutual and reliable identification | • To reassure the patient and make sure that the person taking care of them is a health care professional. |
| Respect for ethical standards and data protection regulations | • A remote medical consultation is considered a medical act. Therefore, all ethical and legal considerations of any doctor-patient relationship apply.• Confidentiality and patient privacy must be ensured. It is recommended to use communication methods that guarantee the highest possible security. |
| Transparency in information | • Information provided to the patient should be concise, transparent, understandable, and easily accessible, using clear and simple language.• Patients should be able to physically access all information provided over the phone. If requested in writing, it should be provided to them. |
| Informed consent | • The option to receive remote care must be accepted and agreed upon with the patient. Any resulting decisions must also be mutually agreed upon and not imposed by any party.• There should be an implicit willingness and, at least, verbal consent from the patient, responsible family member, or legal guardian, as appropriate. This must be documented in the medical record.• The physician must be fully independent and free to choose or decline a telemedicine visit. Upon acceptance, the physician assumes full professional responsibility for the medical act and its implications, particularly regarding diagnosis, advice, treatment options, and direct medical interventions. Therefore, for certain situations (e.g., diagnosing HIV or administering non-oral treatments), the patient may need to be referred for an in-person consultation.25 |
| Documenting in the health record | • The method of teleconsultation used, the prescribed treatment, and any recommendations provided should be documented in the patient's health record. |
On the other hand, patient absenteeism from scheduled consultations is one of the main problems in Specialized Care Centers, generating economic and productive inefficiencies.28 To mitigate this issue, each center's specific situation should be analyzed to determine the factors contributing to absenteeism, categorize them, and implement appropriate corrective measures.29,30 Sending mobile phone reminders or establishing appointment management protocols that include mechanisms for recovering missed activities seem to be effective measures to counteract its effects.28
In the specific context of STI consultations, absenteeism can result in untreated diseases, with repercussions not only for individuals but also for public health. According to Article 61.4 of the Medical Code of Ethics: “the doctor must inform the patient with a sexually transmitted infection about the potential risk of transmitting it and the unavoidable obligation to communicate this fact to those with whom they have had or will have sexual relations. Furthermore, the doctor must warn the patient that if they do not voluntarily assume this duty of informing, the professional could be exempted from the duty of confidentiality in cases of serious risk to third parties”.11 Therefore, even if the patient does not attend the scheduled appointment, when professionals detect cases related to notifiable diseases or epidemic outbreaks, they are required to report them to the health authorities. In such cases, the doctor will record the circumstances in the clinical history. Both professionals and health authorities must make sure that this information does not cause discrimination or harm of any kind to the affected individuals. The limits of informed consent in an STI consultation are indicated in Annex 2.2.
Legal concerns in managing STIs in minorsIt is assumed that during childhood, minors are unable to make certain complex decisions because, due to developmental, educational, and other reasons, they lack sufficient resources to understand the impact and consequences of these decisions, as well as to comprehend possible alternatives. For this reason, minors’ decision-making ability is legally limited.
Adolescence is a transition phase from childhood to adulthood. It is a period marked by significant physical, social, and emotional changes, including the development of secondary sexual characteristics, and for many, a stage in which their sexual orientation is shaped.
Health care professionals involved in adolescent care should integrate sexual education into their clinical practice. This includes discussing risk-reduction behaviors: alternatives such as abstinence, the systematic and correct use of condoms, and the reduction in the number of sexual partners should be part of the patient interview.
The protection of privacy and confidentiality of health data are fundamental principles in the management of any patient, but they take on even greater importance in the management of STIs in minors. The ability to guarantee a private conversation between the adolescent patient and the health care professional is an important aspect that must be guaranteed during the medical visit.
Patients should be informed about the laws that protect their privacy and confidentiality16,17,20 (Annex 3.1), as well as their limitations. In other words, situations where, due to the minor's age, degree of maturity, or the seriousness of the case, it will be necessary to involve, and sometimes obtain consent from, the legal guardians or representatives.
Managing STIs in minors aged 12–16Minors aged 12–16 are considered legally minors in health care. As long as they are considered emotionally and affectively mature and capable of understanding the situation, a patient of this age has the right to be heard, and their opinions should be duly considered based on their age and maturity. For this purpose, they should receive information in comprehensible language, in accessible formats, and adapted to their circumstances. However, their legal guardians or representatives will give consent for decision-making on their behalf.14
The presence of an STI in a minor under 16 years of age should be considered a possible marker of sexual abuse.
Managing STIs in patients aged 16–18In the case of a minor who is emancipated or is 16 years old and has not been legally declared incapacitated nor considered incapable (in the opinion of the health care provider), the minor would be the sole holder of the right to informed consent regarding tests, receiving results, and administering treatments, without the need to inform legal guardians or obtain their consent on the minor's behalf.
However, if the medical decision to be consented to involves a serious risk to the minor's health or life (again, at the discretion of the health care provider), consent will be given by the parents or guardians after hearing and considering the minor's opinion.
Suspected sexual assault in a minorIn general, any act that violates another person's sexual freedom without their consent is considered a sexual assault. Consent will only be understood to exist when it has been freely expressed through actions that, given the circumstances of the case, clearly indicate the person's will.31
The Criminal Code specifically addresses sexual assaults on minors under 16 years of age, considering not only any sexual act performed with the minor as assault but also sexual acts that the minor performs with a third party or on themselves at the instigation of the perpetrator (Annex 3.3).32
However, it will not be considered sexual assault if there is free consent from the minor under 16 years of age and the perpetrator is close to the minor in age and level of physical and psychological development.
The heads of Health Services and the health care personnel within them are especially obligated to report to judicial authorities any fact they become aware of in the course of their work that may be a crime, especially when it involves minors under 16 years of age.
Management of victims of sexual assaultManagement of victims of recent sexual assault (≤10 days) is usually centralized in the ER of reference hospitals.33–35 However, due to our specialization in Venereology and the continuity of care shifts during residency, dermatologists must become familiar with the relevant medical-legal procedures.
Annex 4.1 provides a legal introduction and the legal framework governing such procedures, which apply regardless of whether the victim files a complaint.36,37
Clinical and medico-legal assessmentThe medical and forensic evaluation should be performed jointly in a single session, in a location that ensures the safety and privacy of the victim and in the presence of an authorized companion, if desired. The patient must give written consent for the various procedures (Annex 4.2). This will allow the court on duty to be notified for the immediate transfer of the forensic physician and the Judicial Police to facilitate the in situ filing of a complaint. If there is no consent, only a clinical evaluation will be performed, and a medical injury report will be issued.
Medical historyThe clinical interview should include:
- -
Personal history: medical, substance use, previous violence.
- -
Circumstances of the assault: place, time, presence of witnesses, etc., with descriptive data and direct quotes in quotation marks.
- -
If there have been or are any symptoms indicative of substance use or administration38: amnesia, alterations in consciousness, motor skills, speech, vision, etc.
- -
Actions taken after the assault: hygiene, food or drug intake, etc.
There are 2 types of samples depending on their purpose:
- -
Medico-legal samples: These take priority over the rest and are collected by the forensic doctor (if their presence is not possible, health care personnel are authorized to collect them36). They must be recorded on a specific form and are subject to a strict chain of custody to make sure they are not tampered with, which could invalidate them.
- 1.
For DNA identification:
- a)
Indubitable specimens: known origin, belonging to the victim.
- b)
Dubious samples: unknown origin, pending identification.
- 2.
For chemical–toxicological studies.
- 3.
Images.
- 4.
Other evidence.
- -
Clinical–microbiological samples: for diagnosing STIs and pregnancy. They must be recorded in the medical history. Later, they may also be used for medico-legal purposes.
Table 337 lists all the samples that must be collected. General guidelines for collection are illustrated in Annex 4.3. After this procedure, the victim may clean themselves.
Medical-legal, clinical–microbiological, and other evidence to be collected during physical examination in cases of suspected recent sexual assault, after patient consent.
| Medical-legal samples | |||
|---|---|---|---|
| For medico-legal biological-genetic studies (follow order established) | Oral cavity | Up to 48h post-sexual assault | (a) Semen search: gently swab under the tongue, on gums, teeth, and palate with 2 sterile swabs.(b) Vigorous mouth rinse with sterile saline solution (SSF), stored in a sterile container. Keep refrigerated.(c) Indubitable sample: 2 swabs rubbing the inner cheek area. |
| Body surface | Up to 72h post-sexual assault | Swab possible traces of semen, blood, saliva, or others with 2 sterile swabs. | |
| Anal margin | Up to 72h post-sexual assault | Swab the skin-mucosal surface with 2 moistened sterile swabs. | |
| Anorectal canal | Up to 72h post-sexual assault | Insert 2 moistened sterile swabs to a depth of 3cm up to 5cm. | |
| Pubic hair | No time limit specified | Comb over white paper. Send the comb and paper. | |
| External genitals | No time limit specified | Swab the entire vulvar region with 2 moistened sterile swabs. | |
| Internal genitals | Up to 7–10 days post-sexual assault | Valid regardless of activities post-assault (showering, urinating, sexual activity):(a) Swab the vaginal walls with 2 dry sterile swabs.(b) Insert 2 dry sterile swabs into the cervix.(c) Vaginal wash with 10mL of sterile saline solution, poured into a sterile container. | |
| Fingernails | No time limit specified | Cut the distal part of all nails or clean with 2 sterile swabs. Store samples from each hand separately. | |
| Clothing | No time limit specified | Wrap each item separately in paper and place in independent paper bags. | |
| Venous blood | No time limit specified | Indubitable sample: 2.5mL of blood in an EDTA anticoagulant tube. Keep refrigerated. | |
| For chemical–toxicological studies (if intake has been reported or suggested | Urine | Up to 5 days post-sexual assault | Preferred sample: Collect as much as possible in a sterile container. Keep refrigerated (or frozen if analysis is delayed>24h). |
| Venous blood | Up to 48h post-sexual assault | 2 tubes of 5mL: one with sodium fluoride as a preservative and potassium oxalate as an anticoagulant, the other with EDTA. | |
| Hair | Yes>5 days | Wait 4–6 weeks after the aggression. Cut a 7mm diameter lock from the occipital area as close to the scalp as possible. Attach to paper, marking the proximal and distal ends. Store at room temperature. | |
| Clinical–microbiological samples | ||
|---|---|---|
| Samples must be drawn from all locations exposed during sexual practices, as well as from any ulcers and cutaneous-mucosal wounds.These samples should be collected immediately after the medical-legal samples are drawn, and for each specific location exposed during the assault. | ||
| Urine | 5–10mL in a sterile container; ideally after 2h without urinating. | - First void: PCR for STIs (liquid Amies).39- Midstream: Urine culture.- Pregnancy test. |
| Pharynx (if oral penetration) | Collect BEFORE mouthwash. Swab the entire oropharynx with 2 dry sterile swabs. | - PCR for STIs (liquid Amies).- Culture for N. gonorrhoeae (gel Amies). |
| Vagina | No lubricant. Collect exudate if abundant or swab the posterior vaginal fornix with 2 dry sterile swabs. | - PCR for STIs (liquid Amies).- Gram stain and microbiological culture (gel Amies). |
| Cervix | No lubricant. Swab the cervix and insert into the cervical canal with 2 dry sterile swabs. | - PCR for STIs (liquid Amies).- Gram stain and microbiological culture (gel Amies). |
| Urethra (penis) | No lubricant. Collect exudate if abundant or swab the interior of the urethra with 2 dry sterile swabs. | - PCR for STIs (liquid Amies).- Gram stain and microbiological culture (gel Amies). |
| Anorectal | No lubricant. Collect exudate if abundant or swab the interior of the anal canal (3cm deep) with 2 dry sterile swabs. | - PCR for STIs (liquid Amies).- Culture for N. gonorrhoeae (gel Amies). |
| Oral or genito-anal ulcers | Wash with sterile saline (SSF).Swab the ulcer bed with a dry sterile swab. | - PCR for ulcer-causing STIs (Herpes, Treponema pallidum, LGV, CMV, MPOX, Haemophilus ducreyi) (liquid Amies). |
| Conjunctiva (if exudate) | Collect exudate if abundant or swab the free edge of the eyelid with 2 dry sterile swabs.Ophthalmologist evaluation | - PCR for STIs (liquid Amies). |
| Venous blood | 1 tube, 5mL without anticoagulant. Repeat according to window periods for seroconversion. | - STI serologies: HIV, syphilis, hepatitis A, B, C, HSV. |
| Images |
|---|
| Guarantee confidentiality (cover the face).Obtain victim's consent for each image individually before storage.Explain that the images could be shown in court if required and that the other party will also have access to such images. |
SSF: sterile saline solution; EDTA: ethylenediaminetetraacetic acid; PCR: polymerase chain reaction; STIs: sexually transmitted infections; LGV: lymphogranuloma venereum; CMV: cytomegalovirus; MPOX: monkeypox; HSV: herpes simplex virus; HIV: human immunodeficiency virus.
Without prejudice to any life-threatening injuries that must be treated immediately, once the samples are collected, medical treatment and necessary preventive measures should be implemented:
- -
Treatment of physical injuries.
- -
Tetanus prophylaxis: In the presence of lacerated-contused wounds, depending on their characteristics.
- -
Pregnancy prevention:
- a)
If the victim was not previously pregnant or is not using an effective contraceptive method, after obtaining consent, pregnancy prophylaxis can be provided by the Gynecology Service.
- b)
The next menstrual period should be confirmed, or a pregnancy test should be repeated 2–3 weeks later.
- c)
The victim must be informed that, in the event of pregnancy resulting from the sexual assault, they are legally entitled to an abortion.
- -
Prevention of STIs1,7,40: post-exposure prophylaxis (PEP) should be offered for HIV, HBV, gonorrhea, Chlamydia, syphilis, and Trichomonas vaginalis (Table 4) along with follow-up serological tests (Table 5).
Table 4.Post-exposure prophylaxis for STIs. GRADE levels of evidence.
HIV Type of exposure Recommendation Treatment protocol Anal or vaginal intercourse (insertive or receptive) without a condom or improper condom use PEP is recommended (strong recommendation, low-quality evidence) Tenofovir/emtricitabine every 24h+raltegravir every 12h, for 28 days (strong recommendation, moderate-quality evidence). Start preferably within the first 24h and always within 72h. Do not start after 72h (strong recommendation, moderate-quality evidence). Discontinue if the source is confirmed to be HIV-negative (strong recommendation, low-quality evidence). Orogenital intercourse (insertive or receptive) without a condom or improper condom use, with or without ejaculation Individualize PEP (weak recommendation, low-quality evidence) Exposure of other mucous membranes or non-intact skin to potentially infectious fluids (blood, semen, vaginal secretions, any fluid with traces of blood) Exposure to any fluid on intact skin PEP not recommended (strong recommendation, low-quality evidence) Any type of exposure to non-infectious fluids (urine, feces, saliva, sweat, vomit, tears, nasal secretions, as long as they do not contain traces of blood) HBV Victim's vaccination status Recommendation Unknown or incomplete Administer 1 dose of HBIG and initiate/complete vaccination within the first 24h (strong recommendation, moderate-quality evidence). Complete Act based on HBs Ag titers, if available within 24h:• HBs Ag≥10mIU/mL: protected. No need for PEP.• HBs Ag<10mIU/mL or not available within 24h: 1 dose of HBIG+vaccination (strong recommendation, moderate-quality evidence). Other STI Infection Recommendation and treatment protocol Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis - Single-dose regimen for convenience: IM ceftriaxone 500mg+azithromycin 1g orally+metronidazole 2g orally (strong recommendation, moderate-quality evidence). CDC7: IM ceftriaxone 500mg+doxycycline 100mg orally every 12h for 7 days+metronidazole 500mg orally every 12h for 7 days. Syphillis PEP with penicillin G is ill-advised HCV PEP is ill-advised. Serological follow-up for early treatment (strong recommendation, very low-quality evidence). HPV CDC7: First vaccine dose if the victim is under 26 years old and unvaccinated. CDC: Centers for Disease Control; IM: intramuscular; HBIG: hepatitis B immunoglobulin; HBs Ag: hepatitis B surface antigen; PEP: post-exposure prophylaxis; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; HPV: human papillomavirus.
Adapted from the Expert Group of the National AIDS Plan Secretariat (SPNS), AIDS Study Group (GeSIDA), Spanish Society of Medicine and Occupational Safety (SEMST), et al.40
The management of a victim of suspected sexual assault requires the completion of various documents, detailed further in Annex 4.4:
- -
Clinical history and medical report.
- -
Injury report.
- -
Standardized form for collected samples and chain of custody.
Before discharge, a follow-up care plan must be established:
- -
Coordination with other health care resources, such as Primary Care, Dermatology, Gynecology, Internal Medicine, Preventive Medicine, Psychiatry, and Mental Health, depending on the needs of each case.
- -
Coordination with other regional services for comprehensive victim care: 24-h crisis centers, phone line 016, social services, etc.
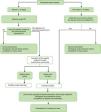
The general action plan is outlined in the flowchart in Fig. 2.
Flowchart for action in case of suspected sexual assault.
Adapted from the basic guidelines of the Spanish National Health System for the management of sexual violence.7.
STIs have certain medico-legal particularities due to their infectious-contagious nature, their mode of transmission, and potential conflicts between the patient's right to confidentiality and public health protection. Understanding the legal framework surrounding these issues and applying it in daily clinical practice is of paramount importance, especially as their incidence increases. This document aims to provide both theoretical and practical information to facilitate the care process and guide physicians in their actions.