UV filters are used daily by millions of people. Not all of these filters, however, are 100% biodegradable, and many wastewater treatments plants are ill-equipped to filter them properly. As a result, UV filters are increasingly reaching the environment. Various types have been detected in soil, continental water, oceans, and numerous organisms, including algae, corals, fish, mammals, and even land birds. In addition, some filters, benzophenone-3 and octocrylene in particular, are toxic to these organisms. Toxic effects include coral bleaching and interference with metabolic, enzymatic, and reproductive activities in practically all organisms. Preliminary data suggest that UV filters may be bioaccumulating in humans, as they have been detected in urine and breast milk. It should be noted, however, that research into the environmental impact of UV filters holds challenges and limitations.
Los filtros ultravioleta (UV) se han convertido en compuestos de uso diario para millones de personas. Sin embargo, algunos de ellos no son biodegradables al 100% y las plantas de tratamiento de aguas residuales muchas veces no son capaces de filtrarlos correctamente. Todo ello está llevando a su diseminación ambiental y a la detección de distintos filtros UV en el suelo, las aguas continentales, los océanos y en múltiples organismos (algas, corales, peces, mamíferos, incluso aves terrestres). Además, algunos filtros UV, especialmente la benzofenona-3 y el octocrileno se han mostrado tóxicos en estos organismos. Entre sus efectos tóxicos destacamos el blanqueamiento de los corales y problemas metabólicos, enzimáticos y de capacidad reproductiva en prácticamente cualquier organismo. Existen datos preliminares sobre la posible bioacumulación de estos filtros UV en humanos, al detectarse en muestras de orina y leche materna. Sin embargo, el estudio del impacto medioambiental de los filtros UV presenta muchas limitaciones.
Ultraviolet radiation (UV) is a fundamental risk factor for the development of nonmelanoma skin cancer and it is also implicated in the pathogenesis of melanoma.1–6 UV radiation is also associated with premature skin aging.4
Photoprotection is the main preventive strategy for reducing the risk of developing skin cancer and achieving healthy aging. Given that skin cancer is the most prevalent cancer in the Caucasian population, the importance of photoprotection is beyond question.7 In recent years, the use of sunscreens as become widespread as an essential component of photoprotection, particularly in Western countries.8 Sunscreens can be classified as organic, inorganic, and biological according to their mode of action.9 Organic filters are the most widely used and can protect against both UVA and UVB radiation.1,10 The sunscreen formulas available on the market usually include a mixture of organic and inorganic filters with 20 or more components to ensure sufficiently broad spectrum coverage.11,12 In addition, these sunscreens include other types of product such as perfumes, hair tints, and plastics.
In recent years, studies have reported accumulation of UV filters in different ecosystems and the toxic effect these can have on many of them. This is due not only to the widespread use of sunscreens in certain environments such as beaches but also because some filters are not readily eliminated with habitual techniques used in wastewater treatment plants, thus contaminating water released to rivers and seas.13–16
Substantial quantities of UV filters such as benzophenone 3 (BP-3) and ethylhexyl salicylate (EHS) have been detected in different water sources and sediments (rivers, beaches, seas, lakes, etc.) throughout the world.17 Moreover, some studies suggest that the UV filters can persist for a long time in water and sediments, and that seas and currents can transport them over large distances.18,19 Also, as might be expected, many of these compounds such as octocrylene (OC) and BP-3 are also found (sometimes at very high concentrations) in living organisms such as algae, corals, fish, and even mammals. Thus, there is a growing body of evidence on the toxic effect of UV filters in these organisms, with the potential to cause problems in the food chain20 and therefore also impact the environment in general.14
Currently, we should also be keenly aware of the effects of global warming. On one hand, the mean temperature of water is increasing, with a negative impact on many ecosystems, particularly marine ones. On the other, there is an increasing number of sunny days throughout the world, and this may lead to an increased use of UV filters, with the subsequent accumulation of sunscreen products and greater toxicity for affected ecosystems.20
In view of the above, the main objective of this study was to perform an exhaustive and critical review of the evidence available on the environmental impact of UV filters.
MethodsWe undertook an extensive narrative review of the literature from different databases to analyze the existing evidence about the impact of UV filters on the environment. The following inclusion criteria were defined: (1) Articles that analyze UV filters (regardless of type of filter and composition); (2) articles that report the impact of these products on the environment (flora, fauna, food chain, water, etc.); (3) no restrictions were applied for the outcome variable; and (4) any study design was accepted, except for opinion articles. Articles published in any language other than English or Spanish were excluded.
The following literature databases were analyzed from their creation through July 2021: Pubmed, Dialnet, Advanced Google, and Trip. Different search strategies were applied was for each one of these, in which both controlled and free language with key words such as photoprotection, environment, future, or filters UV were used.
The articles were selected in 3 phases, which were conducted by a single reviewer. In the first step, the citations retrieved by the search strategies were screened by reading the title. All those that did not meet the inclusion criteria were eliminated. The remaining citations were then screened using the abstracts. Finally, the selection process was completed with a detailed reading of each article. Those that met the inclusion criteria were definitively accepted. Finally, a secondary search was performed of articles included through reading of the reference list of the retrieved articles.
The same reviewer who selected the articles compiled data on the studies included. The quality of the studies included was assessed using the 2011 Oxford scale.21 The evidence and the findings were tabulated.
A qualitative synthesis of the compiled data was then undertaken.
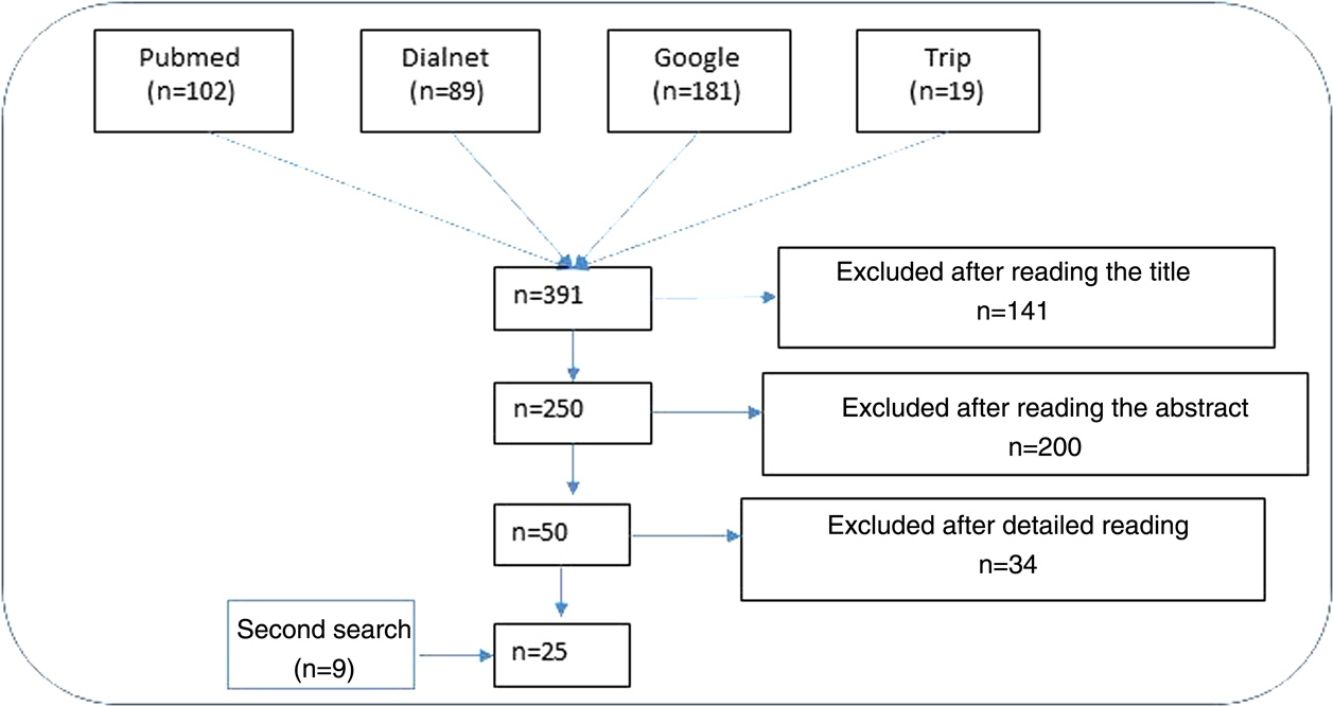
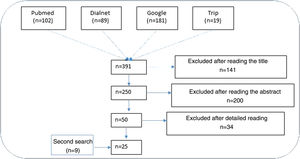
ResultsNarrative review of the literatureIn total, 391 articles were retrieved from the literature databases using the search strategies; of these, 141 were eliminated in the first screening (Fig. 1). On reading the abstracts, a further 200 were eliminated. Finally, on reading in detail the articles that passed through the first 2 screening cycles, an additional 34 articles were excluded, leaving 25 articles (9 from the secondary search).11,15,22–44
We will now describe the main findings of the studies included in the review and other data related to these articles.
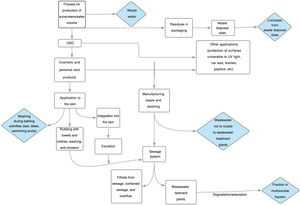
Impact of ultraviolet sunscreens on the environmentTables 1 and 2 show the characteristics, findings, and main conclusions of the 25 studies analyzed in this review.11,15,22–44Fig. 2 shows the main pathways by which UV filters can enter the environment.
Main characteristics of the studies.
| Study/design | Organism/environment | Species | Environment | UV filters | Duration of exposure | Variables | Quality |
|---|---|---|---|---|---|---|---|
| Araújo et al.22 in vitro | Sole (larva) | Solea senegalensis | Portugal | 4-MBC | 96h | MortalityGrowthDeformitiesAlterations in behavior and biomarkers | Oxford 4 |
| Araújo et al.43 in vitro | Fathead minnow | Pimephales promelas | Osage Catfisheries Inc. Osage Beach, USA | EHMC | 14 days | Sexual metabolism (hormonal transcription, secondary sexual characteristics, gonadal histology) | Oxford 4 |
| Danovaro et al.11 in vivo and in vitro | Coral (hard) | Acropora spAcropora pulchra | Tropical regions: Atlantic, Indian, and Pacific ocean and Red Sea | BMDBMBP-3EHS4-MBCOCEHMC | 48h96h | BleachingExpulsion of coral mucous (zooxanthella and coral tissue) | Oxford 3b |
| Downs et al.23 in vitro | Coral (planula) | S pistillataP damicornisA cervicornisM annularisM cavernosaP asteroidesP divaricata | Hawaii, USAAmerican Virgin Islands | BP-2BP-3 | 24h | MortalityDeformityBleachingExpulsion of mucous (zooxanthella and coral tissue)DNA damage | Oxford 4 |
| Du et al.40 in vitro | Fresh water microalgae | Chlorella vulgarisDaphnia magnaBrachydanio rerio | Hydrobiology Institute, Wuhan, China | BP-3BP-4BP-3+BP-4 | 96h | MortalityGrowth | Oxford 4 |
| Fel et al.24 in vitro | Coral (hard) | Stylophora pistillata | Aqaba Gulf, Red Sea | OCBMDBM | >100h | Bleaching | Oxford 4 |
| Fong et al.42 in vitro | Zebra fish (embryos) | Danio albolineatus | Hong Kong | BP-2 | 5days | Neurological development and facial formation | Oxford 4 |
| Grabicova et al.44 in vitro | Rainbow trout | Oncorhynchus mykiss | Lower Bohemia, Czech Republic | PBSA | 21 and 42 days | Enzymatic changes and biochemical parametersChanges in the liver and gonads | Oxford 4 |
| Gago25, doctoral thesis (3 studies) | Aquatic ecosystem (river water, river fish, and marine dolphins) | Luciobarbus sclateriCyprinus carpioPontoporia blainvillei (Franciscian dolphin) | Ebro River, SpainGuadalquivir river, SpainBrazilian marine coast | BP-1BP-3OCOD-PABA4-HBEHMC | See doctoral thesis studies | Presence of UV filters in river sedimentsPresence of UV filters in river fish tissue and sea dolphins | Oxford 2b |
| Giraldo et al.26 in vitro | Marine microalgaeMusclesSea hedgehog | Isochrysis galbanaM galloprovincialiP lividus | Vigo Estuary, Spain | OCOD-PABA | 48h | EmbryogenesisGrowth rate | Oxford 4 |
| He et al.27 in vitro | Coral (hard) | Seriatopora caliendrumPocillopora damicornis | TaiwanHong Kong | EHMCOC | 7 days | MortalityTotal reaction of the polypBleaching | Oxford 4 |
| He et al.28 in vitro | Coral (hard)Coral (planula) | Seriatopora caliendrumPocillopora damicornis | TaiwanHong Kong | BP-1BP-3BP-4BP-8 | 14 days | MortalityBleaching | Oxford 4 |
| Liu 201529 in vitro | Golden carp | Carassius auratus | Nanjing, China | BP-1BP-2BP-3BP-4 | From 7 to 28 days | Changes in biomarkersHistological changes | Oxford 4 |
| Mitchelmoreet al.30 in vitro | Aquatic ecosystem (seawater, corals) | – | Hawaii, USA | BP-3HSOCOS | – | Presence of UV filters in seawater and sedimentsPresence of UV filters in coral tissue | Oxford 4 |
| Molins-Delgado et al.15 cross-sectional | Eggs of wild birds | Birds of falcon family (Circus aeruginosus, Falco tinnunculus)Birds of stork family (Ciconia ciconia)Birds of gull family (Chroicocephalus genei, Chrococephalus ridibundus, Gelochelidon nilotica)Birds of duck family (Anas Strepera) | Doñana Natural Park, Spain | BP-1BP-34-HB4-DHBOD-PABAUVP | – | Presence of UV filters in eggs | Oxford 3a |
| Paredes et al.31 in vitro | Marine microalgaeSea hedgehogMusclesOther crustacean | Isochrysis galbanaParacentrotus lividusMytilus galloprovincialisSiriella armata | A Coruña, Spain | BP-3EHMCBP-44-MBC | 48h | Larva sizeGrowth | Oxford 4 |
| Rodríguez López32 in vitro | Marine microalgae | Tetraselmis suecica | A Coruña, Spain | OCBP-3+OC | 144h | Cell growthCell activityPigment concentration | Oxford 4 |
| Schlumpf et al.33 observational prospective | Newborn rats | Long–Evans rat | Zurich, Switzerland | BP-3HS4-MBCEHMCOD-PABABMDBM | 4days | Changes in uterus sizeChanges in body weight | Oxford 2b |
| Seoane34 in vitro | Marine microalgae | Tetraselmis suecica | A Coruña, Spain | BP-3 | 24h | Cell changes | Oxford 4 |
| Sieratowicz 201141 in vitro | Marine microalgaeCrustacean | Desmodesmus subspicatusDaphnia magna | Florsheim, Germany | BP-3EHMC3-BC4-MBC | 72h | GrowthReproductionLength of adults | Oxford 4 |
| Soto and Rodríguez-Fuentes35 in vitro | Sargeant fish | Abudefduf saxatilis | Quintana Roo, Mexico | BP-3OSEHMC | 96h | MortalityEstrogen effects | Oxford 4 |
| Stien et al.36 in vitro | Coral (hard) | Pocillopora damicornis | Oman | OC | 7 days | Metabolic changesChanges in mitochondrial function | Oxford 4 |
| Stien et al.37 in vitro | Coral (hard) | Pocillopora damicornis | Oman | OCMBBTBP-3BMDBMDHHBEHSHSBEMTDBTET | 7 days | Total reaction of the polypMetabolic changes | Oxford 4 |
| Thorel et al.38 in vitro | Marine microalgaeCrustacean | Tetraselmis speciesArtemia salina | AquarHéak Aquaculture, Ars-en-Ré, France | BP-3BEMTBMDBMMBBTEHSDHHBDBTETHSOC | 7 days | MortalityGrowthCell morphology | Oxford 4 |
| Wijgerde et al.39 in vitro | Coral (hard) | Acropora tenuisStylophora pistillata | Burgers Zoo, Arnhem, Netherlands | BP-3 | 14 days | MortalityGrowthPhotosynthesisMicrobiome disruptionsBleaching | Oxford 4 |
Abbreviations: BEMT, bis-bis-ethylhexyloxyphenol methoxyphenyl triazine; BMDBM, butyl methoxydibenzoylmethane; BP, benzophenone; DBT, diethylhexyl butamido triazone; DHHB, diethylamino hydroxybenzoyl hexyl benzoate; EHMC, ethylhexyl methoxycinnamate; EHS, ethylhexyl salicylate; ET, ethylhexyl triazone; HS, homosalate; MBBT, methylene bis-benzotriazolyl tetramethylbutylphenol; OC, octocrylene; OD-PABA, octyl-dimethyl-PABA; OS, octisalate; PBSA, 2-phenyl-5-benzymidazole sulfonic acid; USA, United States of America; UVP, 2-(2-benzotriazolil)-4-methylphenol; 3-BC, 3-benzylidine camphor; 4-DHB, 4,4′-dihydroxybenzophenone; 4-HB, hydroxybenzophenone; 4-MBC, 4-methyl-benzylidene camphor.
Main findings and conclusions about environmental impact of ultraviolet filters from the studies included.
| Study | Findings and conclusions |
|---|---|
| Araújo et al.22 | Exposure of sole larvae to 4-MBC is associated with higher mortality, malformations, behavioral alternations such as swimming less during periods of light, and enzymatic alterations. |
| Christen et al.43 | EHMC has an impact on the sexual development at different levels with antiestrogen action, histological changes in ovaries and testes, and decreased spermatozoids and oocytes. |
| Danovaro et al.11 | Sunscreens that contain parabens, cinnamates, benzophenones, and camphor derivatives contribute to bleaching of hard coral quickly, even at low concentrations, through promotion of viral infections. |
| Downs et al.23 | BP-3 can induce deformities in coral planulae, bleaching, DNA lesions, ossification, and higher mortality rates at higher concentrations of BP-3. |
| Du et al.40 | BP-3 is associated with decreased growth and mortality in dose-dependent fashion in the microalgae studied.The toxicity of BP-4 was low.The toxicity of BP-3+BP-4 was antagonistic. |
| Fel et al.24 | In chronic exposure to toxic substances, only high concentrations of OC and BMDBM were associated with coral bleaching and their effect was less toxic in comparison with zinc oxide, herbicides, and tributyltin. |
| Fong et al.42 | BP-2 was associated with neurological disorders and facial deformities in fish. |
| Grabicova et al.44 | PBSA increases the activity of certain P450 cytochromes, and causes changes in biochemical parameters and in enzymatic activity. However, pathologic changes were not observed in the liver or gonads. |
| Gago Ferrero25 | Presence of traces of residues from different UV filters in river sediment (up to in 95% of samples), some in very high concentrations, such as OC.High concentrations of UV filters (BP-3, OC, and EHMC) in river fish.Prescence of UV filters in sea dolphins in up to 70% of samples.The authors suggest that urban wastewater treatment plants have limited capacity for filtering out UV filters. |
| Giraldo et al.26 | OC and OD-PABA were toxic (developmental and growth abnormalities) in microalgae, muscles, and sea hedgehogs even at low concentrations. |
| He et al.27 | EHMC and OC, depending on the coral, can cause toxicity (above all, at high concentrations) in terms of mortality and whitening, with percentages ranging from 33.3% to 83.3%. |
| He et al.28 | The presence (concentration-dependent) of BP-1, BP-3, and BP-8 is associated with a moderate to high risk for coral conservation. BP-1 and BP-8 were more toxic for corals than BP-3 and BP-4. |
| Liu et al.29 | BPs (BP-1, BP-2, BP-3, BP-4) led to a reduction in activity of antioxidant hepatic enzymes in golden carp (oxidative stress), as well as liver lesions. |
| Mitchelmore et al.30 | The presence of 8 UV filters in sea, marine sediments, and coral tissue.The most detected UV filter was BP-3 and its concentration was higher in areas where more people congregated |
| Molins-Delgado et al.15 | The presence of UV filters in all samples of birds’ eggs.The authors suggest that the mothers become contaminated on ingesting food, because of the limited capacity for urban wastewater treatment plants to remove UV filters. |
| Paredes et al.31 | BP-3, EHMC, BP-4, and 4-MBC were toxic in all species studied, especially 4-MBC and EHMC |
| Rodriguez López32 | OC did not show disruption to cell growth or impact pigment concentration in microalga studied at concentrations between 2.5 and 5mg/LBP-3 inhibits cell growth and reduces the quantity of carotenoids in microalgae.The combination of OC and BP-3 does not lead to changes in cell growth, but its does increase the cell concentration of carotenoids. |
| Schlumpf et al.33 | BP-3, EHMC, and 4-MBC triggered dose-dependent changes in uterus size of ratsThere were no significant changes in body weight |
| Seoane34 | BP-3 led to an increase in growth rate and esterase activity, a decrease in cell size, and abnormalities in photosynthesis |
| Sieratowicz et al.41 | BP-3, EHMC, 3-BC, and 4-MBC were associated with decreased growth but not the production of neonates or with the length of adults |
| Soto et al.35 | Mild or inexistent interaction between UV filters and estrogen receptors of fishNo association with mortality |
| Stien et al.36 | OC was associated with mitochondrial dysfunction in corals and closed polyps |
| Stien et al.37 | Of the UV filters studied, OC, EHS, and BP-3 were associated with different metabolic changes and mitochondrial dysfunction |
| Thorel et al.38 | Of the UV filters analyzed, HS, BMDBM, and OC were associated with higher mortality, with OC the most toxic, HS, BP-3, and EHS associated with decreased growth, and BMDBM, BP-3, HS with changes in cell morphology |
| Wijgerde et al.39 | Semichronic exposure to BP-3 was associated with accelerated mortality, decreased photosynthesis, changes in microbiome, and whiteningIt is suggested that it has an additional effect to that produced by the increase in temperature |
Abbreviations: BMDBM, butyl methoxydibenzoylmethane; BP, benzophenone; EHMC, ethylhexyl methoxycinnamate; EHS, ethylhexyl salicylate; HS, homosalate; OD-PABA, octyl-dimethyl-PABA; PBSA, 2-phenyl-5-benzymidazole sulfonic acid; 3-BC, 3-benzylidine camphor; 4-MBC, 4-methyl-benzylidene camphor.
Most of the studies included were in vitro studies (although there are also some in vivo ones), from different parts of the world, and of moderate quality with a short duration.11,15,33 The environmental impact of UV filters has been studied on different organisms, including microalgae (seawater and fresh water),11,26,31,32,40,41 crustaceans,26,31,34,38,41 molluscs,26,31 corals,23,24,27,28,30,36,37,39 fish,22,25,29,35,42–44 and mammals.25,33 Much of the impact occurs in embryos and larvae and in aquatic environments, particularly marine ones. However, there is also evidence of these products in land organisms (birds and rats).15,33 Although a wide range of filters have been analyzed, BP-311,15,23,25,28–31,33–35,37–41 and OC11,24–27,30,32,36–38 are the ones most widely studied. The duration of exposure to these UV filters was variable, from 24h23,34 to 42 days.44 We highlight the large variation in doses of UV filters analyzed and the characteristics of the studies; this is a major limitation when making comparisons. In general, most of the studies analyzed the effects of UV filters at concentrations of 100μg/L to 5mg/L, while in vitro studies mainly used acute exposure, and a few studied subacute or chronic exposure.24,39 Likewise, the variables analyzed in terms of toxicity are also diverse, with very heterogeneous measures, criteria, and cut-off points applied. The studies included analyzed mortality, growth rates, coral bleaching, embryogenesis and development of larval forms, altered metabolism and endocrine function (many related to reproduction), deformities, etc.
Both marine and freshwater microalgae are organisms that have been studied. We highlight that BP-3, OC, ethylhexyl methoxycinnamate (EHMC), benzophenone-4 (BP-4), 4-methyl-benzylidene camphor (4-MBC), and octyl-dimethyl-PABA (OD-PABA) were associated with growth abnormalities, BP-3, OC, homosalate (HS), and butyl methoxydibenzoylmethane (BMDBM) with greater mortality, and BP-3 with impaired photosynthesis.26,31,32,40,41
The impact of UV filters on corals has been widely analyzed.23,24,27,28,30,36,37,39 The toxic effects, above all of BP-3, OC, EHMC, and BP-4, have been detected in coral reefs throughout the world, with an estimated 14 000 tons of sunscreen (some of which contain up to 10% BP-3) transferred by swimmers to the coral reefs.11,23,30 One of the most studied effects is coral bleaching.11,20,27,28,30,39 Coral bleaching occurs through loss of essential unicellular algae, the so-called zooxanthellae (Symbiodinium species), which live in the tips of live coral in development toward coral polyps. If the algae is lost, there is a loss of color at the outer edges of the polyp giving rise to a bleaching effect. If this phenomenon continues, the coral dies. Bleaching occurs at concentrations of UV filter between 10 and 300μg/L, depending on the filter and the coral species,23,27,28,30,40 within 18–48h of exposure, with complete bleaching after approximately 96h.27,28,39 In the zooxanthellae family, there is also evidence that some UV filters enhance the risk of propagation of latent viral infections that force these organisms into a lytic cycle with subsequent expulsion from the coral polyp.11,23,26,27,31,37 Other studies have demonstrated that UV filters could cause coral ossification, DNA abnormalities and deformities in the coral larvae,23 changes in metabolic and mitochondrial function,36,37 growth abnormalities,39 and changes to the microbioma39 and photosynthesis.39
Both freshwater and sea fish can also be affected by bioaccumulation and toxicity of some UV filters.22,25,29,35,42–44 In the studies included in our review, filters such as 4-MBC, EHMC, benzophenone 2 (BP-2), and 2-phenyl benzimidazole sulfonic acid (PBSA) had a range of impacts on fish, such as higher mortality, malformations, behavioral changes, changes in sexuality, enzymatic abnormalities, and neurological disorders.
Finally, we highlight that there is also evidence of bioaccumulation in terrestrial animals, specifically in birds. A study performed in the Doñana National Park in Spain analyzed the eggs of different species of birds, and detected the presence of different UV filters.15 The authors suggested that the mothers were contaminated on ingesting contaminated food sources, in light of the limited capacity for the wastewater treatment plants to eliminate UV filters,13–16 and the mothers then transferred these products to their eggs.
Impact of UV filters on the food chain and bioaccumulation in humansWhen products with UV filters are used, a part of the many components used in their formulation may be released from their matrix to the surrounding medium, particularly the aquatic medium. This release will depend on the characteristics of the components of the product, the characteristics of the medium, and a series of physical-chemical phenomena derived from interaction between the two. The end result is that the components can remain unaltered in the environment or they become chemically altered or even undergo transformation into other compounds at a faster or slower rate.45 This means that some filters are not biodegradable, or not fully, or that the degradation process is slow, with the possibility of bioaccumulation or biopersistence in the medium.
Once in the medium, the original or transformed components can interact directly or indirectly with the environment and aquatic life (or even terrestrial life).45 Many UV filters, as they are lipolytic, can readily pass through cell membranes and induce physiological changes in cells.16 Thus, regardless of other toxic effects, as commented above, it has been found that some UV filters accumulate in the fat of many marine and freshwater species.16 In theory at least, this means that they can bioaccumulate at the top of food chain, even reaching humans. Although current evidence is limited, some studies have been published in which BP-3 was detected in urine samples from the United States population,46 and BP-3, 4-MBP, OMC, OC, and other commonly used filters have been detected in breast milk.16 A possible association has also been reported between UV filters and uterine leiomyoma,47 and in the motility of several cell lines of breast cancer and lung cancer.48
Another pathway by which UV filters could reach the food chain is through microplastics. Plastics and their residues are one of the main environmental problems at present. During the degradation process, secondary microplastics are formed, with a diameter less than 5mm. The adverse effects on the biota are due to the chemical composition and the chemical products adsorbed on the surface. Such products include sunscreens and pesticides. When marine microorganisms ingest these microplastics, adsorbed contaminants are absorbed in the digestive tract and so could enter humans, at least theoretically, via the food chain. In a study conducted in the Canary Isles, microplastics obtained from tourist beaches had a higher concentration of UV filters compared with those from nontourist beaches.49,50 A greater oxidative stress in the branchia of the species Scrobicularia plana has also been demonstrated when exposed to microplastics that contained BP-3.51
Limitations to future research of the environmental impact of ultraviolet filtersStudy of the effect of UV filters is extremely complex. There is a huge number of UV filters present in very different products, and the media and organisms (ecosystems) with which they interact are very varied. Some of these systems are extremely complex. There is also a wide range of methodologies used without standardization (study design, techniques, toxicity tests used, duration of exposure, study variables, including measures of toxicity outcome, definitions/criteria, concentrations, thresholds, etc.). Furthermore, data are lacking on the long term effect on the environment.
DiscussionThe literature review undertaken has evidenced firstly that UV filters have accumulated in different media, such as water and sediments, and also in different organisms. This is probably due to different factors; one of these is the massive use in recent years of UV filters. It is estimated that 10000 tons of UV filter are produced a year for the global market.12 Some of these filters possess structural and physical-chemical characteristics that hinder biodegradation, with the subsequent risk of bioaccumulation. This accumulation in water may occur directly, for example, with bathers on a beach (in fact, beaches are the areas where the highest concentrations of UV filters are usually found) or indirectly, given the limited capacity of wastewater treatment plants for filtering them out of wastewater.13–16
On the other hand, our review draws attention to the toxic capacity of some of these filters, above all BP-3 and OC in multiple organisms; this is often dose-dependent, particularly in the case of in vitro exposure.22,26–44 In vivo toxicity has also been found, although to a lesser extent.11,15,25 On this point, we want to highlight that study of environmental impact is very complicated (methodological limitations, very complex ecosystems, dynamic phenomena, etc.) and so these results should be treated with caution. However, although the toxicity detected in the field is not very high, taking into account that the use of filters will likely increase, it is possible that we will see greater damage on reaching higher concentrations of UV filters.
In a recent study, blood concentrations of different sunscreens (BMDBM, BP-3, OC, HS, EHMC, and ethylhexyl salicylate) were found to be in excess of 0.5ng/mL after a single application of sunscreen; currently, the clinical implication in terms of carcinogenicity and/or impact on reproduction is not known. Moreover, given that these filters have a long half-life, usually greater than 48h, repeated use means they can be detected in blood up to 17 days after the last application.52,53 Similarly, although UV filters or their derivatives may reach humans via the food chain, to date, there have been no reports of toxic effects.
Faced with this situation, a range of actions are available to ensure use of UV filters with appropriate photoprotection, essential for preventing skin cancer and skin aging, and also free of environmental impact.
One of the first actions would be to set up a regulatory framework, banning compounds that are shown to be toxic for the environment. Along these lines, some of the most harmful UV filters such as EHMC and BP-3 are already banned in some places, such as Palaos or Hawaii, due to the potential deterioration to coral reefs, one of the main tourist attractions for these places.16
In addition, although it is true that in Spain it is only possible to use UV filters listed in Regulation (CE) 1223/2009 of the European Parliament and Council,54 which should also be used according to the indications for each of them, personal care products (PCPs) can include UV filters, but they do not require environmental study, even though they are considered emerging contaminants.
Also, the lack of standardization in terms of concentrations that may be toxic for the environment, or the lack of definition as to what a biodegradable product is or what constitutes protecting the environment or respecting the oceans means that the packaging often includes information that is inadequate, confusing, or even incorrect.
Change is needed urgently, both in the formulation of the product, aiming to minimize the environmental impact while guaranteeing photoprotection, and in the water purification methods of the wastewater treatment plants. Of importance is that manufacturers of the commercial brands of PCPs with UV filters are aware of the potential negative effects of these filters in the environment. Thus, research institutions and the cosmetic industry should make more effort to identify new molecules to use as UV filters, or mixtures or specific concentrations to provide the necessary photoprotection while having a low environmental impact.
Scientific societies, headed by the Spanish Association of Dermatology and Venereology, should continue in their efforts to raise awareness of the importance of photoprotection. Adequate photoprotection strategies should be multifactorial rather than relying on a single intervention. For example, using photoprotective clothing and swim gear, applying sunscreen to areas exposed to sunlight, limiting exposure times, and taking care with reflective surfaces should all be considered. It should be highlighted that the findings described above do not contradict these photoprotection strategies.
Sunscreens have evolved to better fit the individual needs of individuals, taking into account their age, skin type, presence or risk of diseases, as well as different activities performed by these people.20 It is to be hoped that sunscreens will be improved with increasingly environment-friendly formulations. For this, the strength of evidence, standardization of procedures, and regulations will need to be improved.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We thank Marta Barluenga Badiola, the resident scientist at the National Science Museum, CSIC, for her critical reading and review of the manuscript.