Guselkumab is a fully human monoclonal antibody that targets the p19 subunit of interleukin 23. It has been approved to treat moderate-to-severe psoriasis and/or psoriatic arthritis among the adult population. Despite the recommended dose of a 100mg subcutaneous injection on weeks 0 and 4, prior evidence from former clinical trials and real-world data suggests that the flexibility of guselkumab treatment allows for longer intervals between doses with significant pharmacoeconomic repercussions.1
The objective of our study was to conduct a pharmacoeconomic study of guselkumab, assessing its efficiency profile and persistence in patients with plaque psoriasis in the routine clinical practice.
This was a multicenter, observational, and retrospective study conducted among 6 hospitals from the Spanish autonomous community of Andalusia. A total of 168 patients with moderate-to-severe psoriasis were treated with guselkumab 100mg, with a dosing regimen that was left to the prescribing physician's criterion and based on the accessibility conditions of the corresponding hospital. Treatment response was assessed using PASI, BSA, pruritus VAS, and DLQI over a period of 156 weeks. Additionally, a pharmacoeconomic study compared the hospital pharmacy dispensations and guselkumab technical specifications sheet. Data were expressed as mean ± standard deviation for the continuous variables and number and percentage for the categorical ones. The Shapiro-Wilk test was used to check data normality, and the Wilcoxon test to analyze potential differences across various time periods. The estimated retention rate was calculated using Kaplan-Meier curves. All analyses were performed using GraphPad Prism 8.0.0 (California, United States, www.graphpad.com).
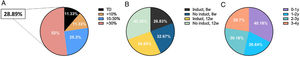
The study population included a total of 168 patients who, after, at least, 2 doses of guselkumab, started showing significant improvements in disease severity parameters compared to baseline (Table 1). In our population, the pharmacoeconomic study conducted confirmed a reduced overall spending by 28.9% compared to the technical specifications sheet (Fig. 1A-C). Specifically, 11.3% of the patients received the dose according to the technical specifications sheet, while 11.3% saved less than 10%, 25.3% between 10% and 30%, and 52% saved more than 30%. Regardless of the prescribed dosing regimen, cost savings ranged from 26.8% up to 42.3%. Over time, the rates of cost savings were 40.2%, 26.6%, 30.2%, and 28.7% within the 1st, 2nd, 3rd, and 4th years, respectively. After 156 weeks on guselkumab, the estimated overall retention rate was 83% (including all-cause treatment discontinuation), while 95.7% was due to lack of efficacy, or safety issues. In patients with special and difficult-to-control locations, retention rates were even higher at 92% and 97%, respectively.
Sociodemographic characteristics of our population.
| Overall population | |
|---|---|
| Patients (n) | 168 |
| Age, (SD) | 50 (14.5) |
| Progression of psoriasis, (SD) | 22 (13.7) |
| Gender, % (n) | |
| Woman | 47.6 (80) |
| Man | 52.3 (88) |
| BMI, (SD) | 29 (5.8) |
| Comorbidities, % (n) | |
| Dyslipidemia | 52.3 (88) |
| Obesity | 17.8 (30) |
| AHT | 22 (37) |
| Psoriatic arthritis | 16.6 (28) |
| Diabetes | 18.4 (31) |
| Depression | 13 (22) |
| Non-alcoholic fatty liver | 13 (22) |
| Dosage (100 mg) | |
| Induction + 8 weeks (technical specifications sheet) | 71 (86) |
| Without induction + 8 weeks | 11.5 (14) |
| Induction + 12 weeks | 2.4 (3) |
| Without induction + 12 weeks | 14.8 (18) |
| Severity Indices, (SD) | |
| PASI | 11.2 (7) |
| BSA | 18.1 (15) |
| Pruritus VAS | 5.5 (2.6) |
| DLQI | 14.4 (6.7) |
| Previous systemic therapy | 1.8 (0.6) |
| NB-UVB | 33.9 (57) |
| CyA | 6.7 (129) |
| MTX | 65.4 (110) |
| ACI | 25 (42) |
| DMF | 16 (27) |
| Previous biological therapy, (SD) | 2.2 (1.5) |
| Anti-TNF | 168 |
| Anti-IL-17 | 80 |
| Anti-IL-12/23 | 75 |
| Previous biological therapy, % (n) | |
| 0 | 10.7 (11) |
| 1 | 41.1 (42) |
| 2 | 24.5 (25) |
| ≥ 3 | 23.5 (24) |
ACI, acitretin; AHT, arterial hypertension; BMI, body mass index; BSA, body surface area; CyA, cyclosporin A; DLQI, Dermatology Life Quality Index; DMF, dimethyl fumarate; GUS, guselkumab; MTX, methotrexate; NB-UVB, narrow-band ultraviolet B phototherapy; PASI, Psoriasis Area Severity Index; SD, standard deviation; VAS, visual analogue scale.
A) Percentage of patients on the dosage recommended by the technical specifications sheet, < 10% savings, 10% to 30% savings, and > 30% savings (TSS: technical specifications sheet). B) Percentage of savings based on the clinical prescription. C) Percentage of savings based on the time the patient had been treated.
The results of our study demonstrate the high efficacy profile of guselkumab in controlling plaque psoriasis in the routine clinical practice. Also, our pharmacoeconomic study highlights the potential for significant cost savings of the individualized dosing regimens, thus leading to a better cost-effectiveness ratio without compromising treatment outcomes. Former studies have shown that guselkumab is more cost-effective than adalimumab, ustekinumab, or secukinumab, and potentially other biological treatments vs psoriasis.2–5
In Spain, the prescription of guselkumab is decentralized, with each Spanish region applying different pharmacoeconomic criteria, resulting in the asymmetric prescription of first-line therapies. In-hospital agreements often result in different dosages that are not consistent with the product technical specifications sheet. For example, Hospital Universitario La Paz, Madrid, Spain recently reported cost savings of 40% and 47%, respectively, in a specific subgroup of naïve patients and super-responders, compared to the technical specifications sheet.6
In conclusion, our study provides valuable information on the efficact profile and pharmacoeconomics of guselkumab in the routine clinical practice. The combination of its clinical efficacy, potential cost savings, and individualized dosing sets guselkumab as an attractive therapeutic option for the management of patients with plaque psoriasis.
We wish to thank Dr. Perez Gil (Hospital Universitario de Valme, Seville, Spain), and Dr. Moreno Suarez (Hospital Universitario de Jaen, Spain) for their contribution to this series.