Basal cell carcinoma (BCC) represents 80% of all non-melanoma skin cancers, and its incidence rate is on the rise.1 Most BCCs are slow-growing, and surgical treatment is often curative. However, occasionally, the tumor can progress locally, invading nearby structures, or systemically, making it unresectable with curative intent through surgery or radiation therapy. These cases are referred to as locally advanced BCC (LA-BCC), and represent 0.8% of all cases of BCC.2
The Hedgehog (Hh) signaling pathway—involved in cell growth and polarization, and inactive in adults—plays a key role in the pathogenesis of BCC when activated through mutations of the Hh signaling pathway molecules.3 Vismodegib and sonidegib inhibit the Hh signaling pathway by binding to the SMO protein and inhibiting tumor growth. Currently, vismodegib is indicated for LA-BCC and metastatic BCC, while sonidegib is only indicated for LA-BCC.2–5 Although both drugs achieve objective response rates over 47% and 60%, respectively, combining them with other therapies leads to better response rates.2–5
We describe the case of a woman with LA-BCC who achieved a complete and long-lasting clinical response by combining systemic treatment (vismodegib) and various physical therapies.
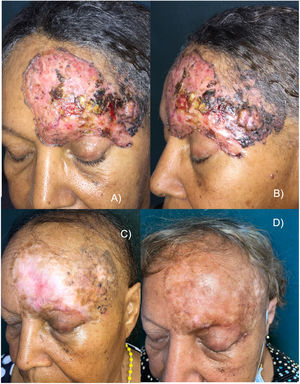
This is the case of a 65-year-old woman with no significant past medical history, but a 30-yesar history of a frontal lesion. Physical examination revealed the presence of a 12cm x 8cm ulcerated nodular tumor on her left frontal region, and areas of pigmentation (Figures 1A and B). A biopsy confirmed the diagnosis of solid pigmented BCC. Imaging modalities confirmed the involvement of the upper eyelid and the internal tarsal region, in contact with the frontal bone without signs of invasion, or distant metastases. Staging ruled out the presence of lymphadenopathy, masses, or megalies. The definitive diagnosis was stage III BCC (T3N0M0).6 The multidisciplinary skin tumor committee ruled out surgery and radiation therapy as curative options, leading to the administration of oral vismodegib 150mg/day. After 2 months, a slow but progressive clinical response was observed, with a reduction in size, gradual disappearance of erythema, and epithelialization of ulcerations. However, some pigmentation persisted. The patient experienced a few typical vismodegib-related toxicities, including grade 2 dysgeusia, grade 1 muscle cramps, grade 2 alopecia, and grade 1 weight loss, which did not require drug discontinuation.
A and B) Appearance of the tumor before starting treatment. Erythematous plaque with ulcerated, crusted areas, and abundant pigmentation. C) After 18 months on vismodegib. Partial tumor response. Alopecia is observed as a characteristic toxicity associated with this drug. D) 30-month follow-up (12 months after vismodegib discontinuation). Complete clinical remission, significant repigmentation, and regrowing alopecia.
Eighteen months into treatment and after achieving a >95% reduction in tumor mass, response stabilization was noted, and vismodegib was discontinued (figure 1C). The residual lesion was biopsied revealing residual nests of pigmented solid BCC. Despite clinical practice guidelines recognize that photodynamic therapy (PDT) is not effective for pigmented BCCs,1 based on the patient's choice, the pigmented residual area was sequentially treated with 4 sessions of PDT (a 3-hour exposure to methyl aminolevulinate, and a dose of 37J/cm2), topical imiquimod (5 days a week for 4 months), and a conservative excision of the last appreciable tumor residue, confirming the persistence of BCC without surgical margin involvement.
Six months after treatment cessation, the histopathological examination confirmed the presence of a relapsed BCC in the left eye external corner that was treated with radiation therapy to the entire field, using photons (80kV) and electrons (6 MeV) for a total dose of 4000 cGy. After treatment, the patient achieved complete clinical remission, which continues 30 months after the completion of radiation therapy. All the biopsies performed after vismodegib therapy were reported as solid BCCs.
Ageusia and muscle cramps disappeared, and alopecia was in the process of regrowth. Notably, partial but significant repigmentation was reported after vismodegib therapy (figure 1D).
The medical literature confirms the efficacy of vismodegib to treat of LA-BCC, both as monotherapy and neoadjuvant therapy, with high remission rates, good functional and aesthetic results, and a favorable safety profile.7–9 In our case, vismodegib achieved a slow but progressive clinical response, sufficient for a complete response to be achieved through the combined use of different physical and surgical therapies, with manageable toxicity.
In the routine clinical practice, the combination of vismodegib and topical therapies, surgery, radiation therapy, or systemic therapies8–10 is a safe and effective therapeutic option. However, more studies are needed to determine which patients will be responders, for how long, and the appropriate sequence of therapy combinations to achieve the best possible results with minimal toxicity.
Conflicts of interestNone declared.