Psoriasis is a chronic inflammatory skin disease that affects between 2% and 3% of the general population.1 Approximately one-third of all patients with this condition are candidates for systemic immunosuppressive therapy, including biologic drugs. Although biologic agents generally have a better safety profile than traditional immunosuppressive therapies, their use has been associated with an increased risk of viral and upper respiratory tract infections.2 Furthermore, psoriasis is frequently associated with cardiometabolic comorbidities, which are risk factors for a poor prognosis in patients with pneumonia caused by infection with SARS-CoV-2.3 Although a few case series and isolated multicenter studies have been reported in the literature, there is still insufficient evidence available on the susceptibility to COVID-19 of patients with chronic plaque psoriasis or on the risks or benefits that may be associated with the use of anti-inflammatory biologic therapies in COVID-19.1–4
We conducted a retrospective observational study of patients diagnosed with moderate to severe psoriasis on biologic therapy (tumor necrosis factor [TNF]-alpha, interleukin (IL) 12/23, IL-17, and IL-23 inhibitors) at the Hospital Universitario Son Espases between March 1, 2020 and January 10, 2021. The objectives of this study were to determine COVID-19 admission rates, incidence, and mortality in patients with psoriasis receiving biologic therapy at our hospital. We calculated 95% CI using the Wald method to compare our findings with the proportions observed in the general population in Spain, based on official data.5,6 We also assessed treatment adherence and the need for withdrawal of treatment. A confirmed SARS-CoV-2 infection was defined as a positive result on either a real-time reverse transcription-polymerase chain reaction (PCR) assay or a serology test positive for IgM and/or IgG antibodies to SARS-CoV-2.
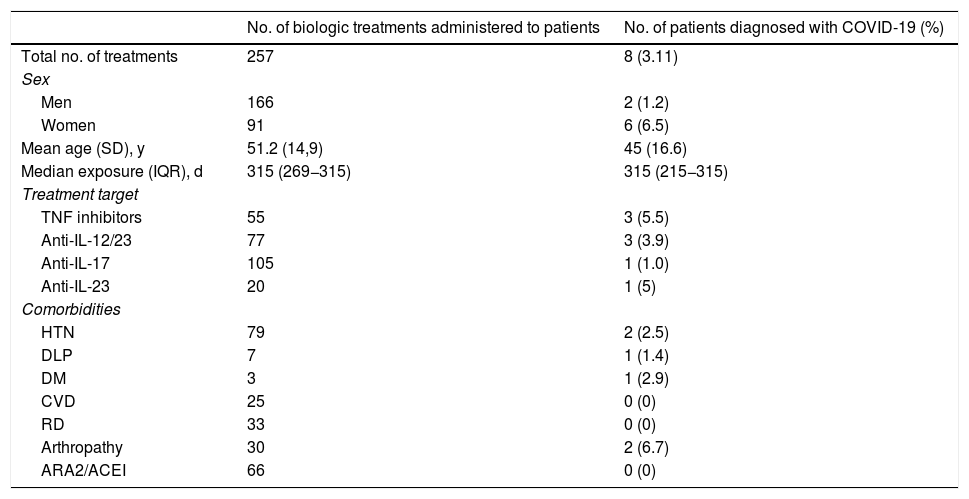
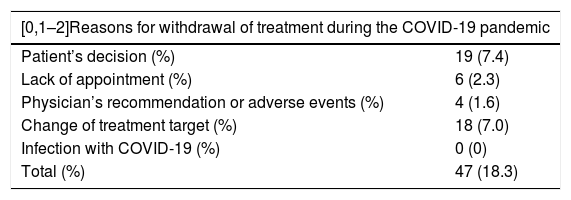
During the period studied, 257 biologic treatments were administered to 239 patients (Table 1). Of these, 166 (64.6%) were administered to men and the mean (SD) age of the cohort was 51.2 (14.9) years. The median time of exposure to the drug was 315 days (interquartile range [IQR] 269−315), with a minimum of 18 days and a maximum of 315 days. After March 1—during the COVID-19 pandemic—treatment was started in 57 patients (22.2%) and was discontinued for various reasons in 47 patients (18.3%) (Table 2). Nineteen patients (7.4%) decided themselves to discontinue treatment with the biologic agent. Biologic therapy was not discontinued due to COVID-19 in any of the 8 patients in the cohort who developed the disease (3.1%; 95% CI, 1.0%–5.2%). The incidence in our cohort was similar to that of the general population in the Balearic Islands, which was 3.35%. The subgroup with the lowest incidence of SARS-CoV-2 infection was the group of patients treated with IL-17 inhibitors; only one of the 105 patients treated with anti-IL-17 biologics contracted the infection. None of the patients in our cohort were admitted to hospital and no deaths from COVID-19 were recorded. Table 1 shows the differences between the COVID-19 cases and the rest of the cohort with respect to comorbidities and the drugs received. The only factor studied that correlated with a higher incidence of COVID-19 was female sex (75% of cases, P = .025).
Comparison of Epidemiological Data, Treatments and Comorbidities of Patients with Psoriasis Being Treated With Biologic Drugs at Our Hospital Who Tested Positive for COVID-19.
| No. of biologic treatments administered to patients | No. of patients diagnosed with COVID-19 (%) | |
|---|---|---|
| Total no. of treatments | 257 | 8 (3.11) |
| Sex | ||
| Men | 166 | 2 (1.2) |
| Women | 91 | 6 (6.5) |
| Mean age (SD), y | 51.2 (14,9) | 45 (16.6) |
| Median exposure (IQR), d | 315 (269−315) | 315 (215−315) |
| Treatment target | ||
| TNF inhibitors | 55 | 3 (5.5) |
| Anti-IL-12/23 | 77 | 3 (3.9) |
| Anti-IL-17 | 105 | 1 (1.0) |
| Anti-IL-23 | 20 | 1 (5) |
| Comorbidities | ||
| HTN | 79 | 2 (2.5) |
| DLP | 7 | 1 (1.4) |
| DM | 3 | 1 (2.9) |
| CVD | 25 | 0 (0) |
| RD | 33 | 0 (0) |
| Arthropathy | 30 | 2 (6.7) |
| ARA2/ACEI | 66 | 0 (0) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ARA2, angiotensin II receptor antagonists; DLP, dyslipidemia; DM, diabetes mellitus; CVD, cardiovascular disease; HTN, hypertension; IL, interleukin IQR, interquartile range; RD, respiratory disease; TNF, tumor necrosis factor.
Reasons for Withdrawal of Treatment During the Study.
| [0,1–2]Reasons for withdrawal of treatment during the COVID-19 pandemic | |
|---|---|
| Patient’s decision (%) | 19 (7.4) |
| Lack of appointment (%) | 6 (2.3) |
| Physician’s recommendation or adverse events (%) | 4 (1.6) |
| Change of treatment target (%) | 18 (7.0) |
| Infection with COVID-19 (%) | 0 (0) |
| Total (%) | 47 (18.3) |
COVID-19 due to infection with SARS-CoV-2 is a new infectious disease with a highly variable course that manifests with a broad clinical spectrum.1–3
We present a descriptive study of SARS-CoV-2 infection in a cohort of patients with psoriasis receiving biologic therapy at the referral hospital for Spain’s Balearic Islands. Our results are consistent with those of earlier studies published in the literature, which found no increased risk of infection with SARS-CoV-2 in patients with this profile and observed low morbidity and low rates of severe disease and deaths in the patients who did develop COVID-19.1–4
It is possible that biologic therapies lessen the cytokine storm described in patients with severe forms of COVID-19, during which excessive amounts of proinflammatory cytokines are produced.3 Of note is the fact that women accounted for 75% of the patients in this cohort who were infected, while the percentage of women who developed COVID-19 in the Balearic Islands was 52%; this finding contrasts with the results of the multicenter studies, in which a male predominance was reported.3–5 Patients treated with IL-17 inhibitors in our cohort had the lowest incidence of COVID-19 (0.96%). The incidence of SARS-CoV-2 infection in our study is very similar to that of the general population in our setting.5,6 Therefore, the patients on biologic therapies were not at higher risk of contracting the infection. Moreover, no cases of severe COVID-19 were reported in this cohort. The main limitation of the study was the small size of the sample and the retrospective study design.
FinancingThis article did not receive any funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gracia-Darder I, Garcías-Ladaria J, Ramos Rodríguez D, Escalas Taberner J. Psoriasis en tratamiento biológico durante la pandemia COVID-19: serie de pacientes del Hospital Universitario Son Espases. Actas Dermosifiliogr. 2022;113:110–112.