Topical and intralesional (IL) treatments may be considered the first-line therapy in patients with hidradenitis suppurativa (HS); however, the evidence supporting their use is limited. The aim of our review is to evaluate the efficacy and safety profile of topical and IL treatments in patients with HS.
Materials and methodsWe designed a systematic review of the current medical literature available following the PICO(T) method. And including all types of studies (Study type [T]) of individuals with HS of any sex, age, and ethnicity (Population [P]) who received any topical or IL treatment for HS (Intervention [I]) compared to placebo, other treatments, or no treatment at all (Comparator [C]), and reported efficacy and/or safety outcomes (Outcomes [O]). Two outcomes were defined: quality of life and the no. of patients with, at least, one adverse event. The search was conducted in the Cochrane Library, MEDLINE, and EMBASE databases; study selection was performed based on pre-defined criteria. The risk of bias was determined in each study.
ResultsWe obtained a total of 11,363 references, 31 of which met the inclusion criteria. These studies included 1143 patients with HS, 62% of whom were women. A total of 10, 8, 6, 2, and 5 studies, respectively, evaluated the use of photodynamic therapy (PDT), glucocorticoids, resorcinol, topical antibiotics, and other interventions. Most articles were case series (n=25), with only five randomized clinical trials (RCTs) and one cohort study. RCTs showed improvement in disease activity with topical clindamycin and botulinum toxin (BTX) vs placebo, and PDT with methylene blue (MB) niosomal vs free MB; however, intralesional triamcinolone acetonide was not superior to placebo. The risk of bias was low in three RCTs and high in two RCTs.
ConclusionThe quality of evidence supporting the use of topical, or IL treatments is low. However, it supports the use of topical clindamycin, PDT, and BTX. Well-designed RCTs with standardized outcomes and homogeneous populations of patients and lesions are needed to support decision-making in the routine clinical practice.
Los tratamientos tópicos e intralesionales (IL) pueden ser considerados como tratamientos de primera línea en pacientes con hidradenitis supurativa (HS), sin embargo, la evidencia apoyando su uso es limitada. El objetivo de nuestra revisión es evaluar la eficacia y la seguridad de los tratamientos tópicos e IL en pacientes con HS.
Material y métodosDiseñamos una revisión sistemática de la literatura siguiendo el método PICO(T). Incluimos todo tipo de estudios (tipo de estudio [T]) que incluyeran individuos con HS de cualquier sexo, edad, y etnicidad (Población [P]), que recibieran cualquier tratamiento tópico o IL para la HS (Intervención [I]) que compararan con placebo, otros tratamientos o no tratamiento (comparador [C]) y reportaran resultados de eficacia y/o seguridad (Outcomes [O]). Dos resultados fueron definidos: calidad de vida y número de pacientes con al menos un efecto adverso. La búsqueda se llevó a cabo en las bases de datos Cochrane Library, MEDLINE y EMBASE; la selección de estudios se realizó de acuerdo con los criterios predefinidos. El riesgo de sesgo se determinó en cada estudio.
ResultadosSe obtuvieron 11.363 referencias de las cuales 31 cumplieron los criterios de inclusión. Estos estudios incluyeron 1.143 pacientes con HS, 62% fueron mujeres. 10 estudios evaluaron la terapia fotodinámica (TFD), ocho glucocorticoides, seis resorcinol, dos antibióticos tópicos y cinco otras intervenciones. La mayoría de los artículos fueron series de casos (n=25), con solo cinco ensayos clínicos aleatorizados (ECA) y un estudio de cohortes. Los ECA demostraron mejoría de la actividad de la enfermedad con clindamicina tópica y con toxina botulínica (BTX) frente a placebo y TFD con azul de metileno (AM) niosomal frente a AM libre; sin embargo, el acetónido de triamcinolona IL no fue superior al placebo. El riesgo de sesgo fue bajo en tres y alto en dos ECA.
ConclusiónLa calidad de la evidencia que apoya el uso de tratamientos tópicos o IL es baja, pero apoya el uso de clindamicina tópica, TFD y BTX. Se requieren ECA adecuadamente diseñados con resultados estandarizados y poblaciones homogéneas de pacientes y lesiones para apoyar la toma de decisiones en la práctica clínica.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition, characterized by recurrent nodules, abscesses, tunnels, and scars. These lesions are often found on the axillae, groins, perianal, perineal, or inframammary regions.1,2 Both onset and progression have been associated with risk factors such as smoking, obesity, and female sex.3,4 HS is relatively common, with an estimated prevalence of 1–4% in European populations,5,6 affecting mainly young adults. Overall, HS is an underestimated health problem that has a significant impact on the patients’ quality of life (QoL).7–9
A wide range of therapeutic options to treat HS are currently available.10 Most studies focus on the efficacy and safety profile in moderate and severe forms of HS. The scientific evidence supporting the use of topical and intralesional (IL) therapies is scarce though. In the routine clinical practice, topical and IL therapies are often first-line therapies to treat early stages of HS. They may also be useful for limited flares in patients already on systemic treatment.10–12 Although a prior Cochrane systematic literature review (SLR),10 summarized the evidence from randomized clinical trials (RCTs), as far as we know, to this date, no SLR on topical and IL treatments for HS has ever been published. Therefore, the aim of our review was to evaluate the efficacy and safety profile of topical and IL treatments for patients with HS.
MethodsWe designed a SLR to assess the efficacy and safety profile of both topical and IL therapies to treat patients with HS. Following the PICO(T) approach, we defined population (P), intervention (I), comparator (C), outcomes (O), and type of studies (T) as part of this SLR conducted in accordance to the clinical practice guidelines provided by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.13 PROSPERO (ID no. CRD42022361114).14
We included studies on individuals with HS of any sex, age, and ethnicity [Population (P)]. The diagnosis of HS could be defined by the treating physician, or meeting the available diagnostic criteria.15
Any type of topical or IL treatment for HS [Intervention (I)] were included. Therefore, both pharmacological and physical interventions could be included. Destructive therapies, such as laser or surgery were considered out of the scope of this review. Studies evaluating a combination of topical and systemic interventions were also excluded. To facilitate interpretation, a total of five intervention groups were pre-defined: PDT, IL corticosteroids, topical resorcinol, antibiotics, and other interventions. No restrictions were imposed on the comparators (C); placebo, absence of intervention or any other medical interventions (topical or systemic) were included. Studies with no comparator were also included, as were studies comparing between different topical, or IL therapies.
Outcome selection was based on the International Dermatology Outcome Measures (IDEOM) consensus document for developing hidradenitis suppurativa results in health measurements.16 Two co-primary outcomes (O) were defined, one regarding efficacy – self-perceived QoL – and the other one regarding safety – number of patients with, at least, one adverse event (AE). To assess QoL, generic (SF-36 or EQ-5D), dermatology-specific (Dermatology Life Quality Index (DLQI), Skindex 29), and HS-specific scales (Hidradenitis Suppurativa Quality of Life 24, HSQoL-24) were included. Pre-defined secondary efficacy outcomes included patient global assessment, pain, pruritus, disease activity, physician global assessment and treatment satisfaction. Outcomes could be assessed on any scale.
All type of studies (T) were included: randomized, case–control, cohort, and case series. To be included, case series needed to report on more than five patients. Narrative reviews were excluded; previous systematic literature reviews were only used for secondary reference identification. Studies were also excluded if the full paper was unavailable, or written in a language other than English, Spanish, or French.
The search was performed across three main scientific databases (Cochrane Library, MEDLINE and EMBASE) on September 29th, 2022 with no time constraints. Due to the difficulty defining the multiple interventions accurately, the search focused on retrieving papers with the study population (patients with HS). To do so, a combination of standardized (MeSH), and free terms were used. Details of the search are shown in the supporting information (SI, Table 1). A ClinicalTrials.gov search was performed using the terms ‘hidradenitis suppurativa’ and the filter ‘complete’.
Results obtained from the search were added to a citation manager (EndNote® X7). Titles and abstracts of all retrieved abstracts were independently screened by two authors (JCP and RHQ). After title and abstract screening, a full text review of the selected studies was performed. Disagreements between the authors were resolved by a referee (FS). Inclusion criteria followed the PICOT structure described above. The reference list of the studies included in the SLR was, then, checked to identify missed papers. No additional search of the international meetings or key journals was performed.
Data curation was independently performed by JCP and RHQ through an ad hoc designed data extraction sheet, based on the ‘Checklist of items to consider in data collection or data extraction’ available in ‘Cochrane Handbook for Systematic Reviews of Interventions’. Discrepancies were settled by consensus.
The risk of bias and the methodologic quality of the studies included were also evaluated. Due to the heterogeneity of these studies (from case series to RCTs), different tools for proper assessment were established: the Cochrane's ‘risk of bias’ tool (RoB2)17 for clinical trials, and the ‘Newcastle-Ottawa Quality Assessment Scale’ (NOS)17 for observational studies.18 All case series were considered of at a high risk of bias.
If heterogeneity was low, a meta-analysis was planned. Outcomes for dichotomous variables were expressed as relative frequencies, while for continuous variables, outcomes were expressed as means and standard deviations. Publication bias analyses were planned and expressed through a funnel plot. Nevertheless, due to insufficient material, these analyses could not be conducted.
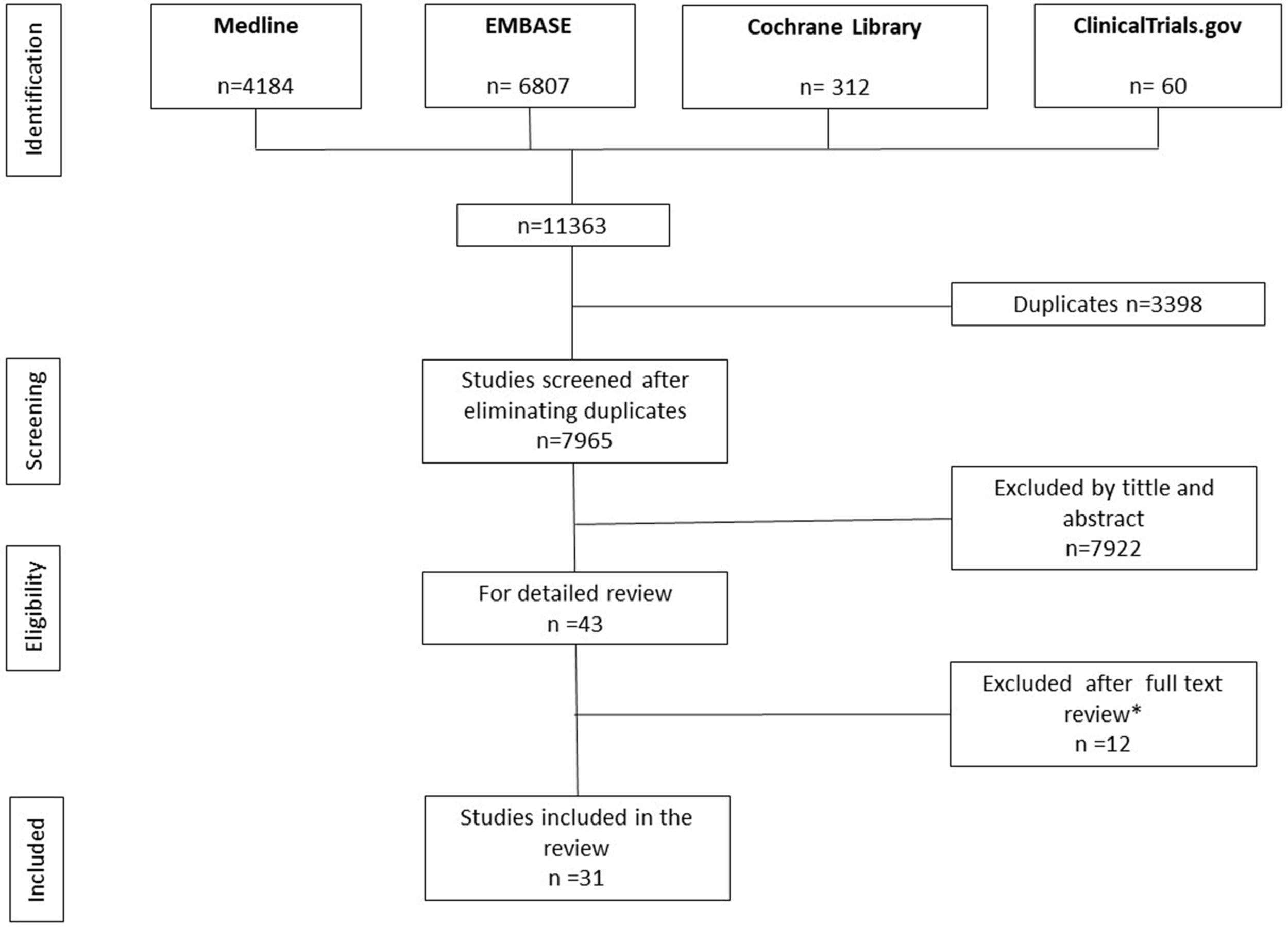
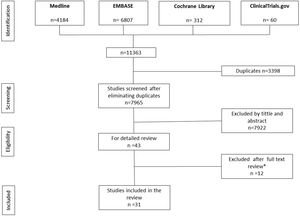
ResultsThe search retrieved a total of 11,363 references, 4184 of which were obtained from MEDLINE, 6807 from EMBASE, 312 from Cochrane Library, and 60 from Clinicaltrials.gov. After duplicate removal and screening by title and abstract, a total of 43 studies were selected for full text review. Of these, 31 fulfilled all inclusion criteria (Tables 1–5),19–49 and 12 were excluded (SI, Table 2)50–61 (Fig. 1). The 31 studies selected reported on a total of 1143 patients with HS, 62% of whom were women. The mean age of participants was 34.1 years old. The mean number of participants per study was 36.2, ranging from 520,21 to 131,37 and the mean follow-up was 113.2 days. Most patients had chronic HS with a mean course of the disease of 11.7 years, and moderate severity (21.4% were Hurley I and 62.9% Hurley II) (SI, Tables 3 and 4).
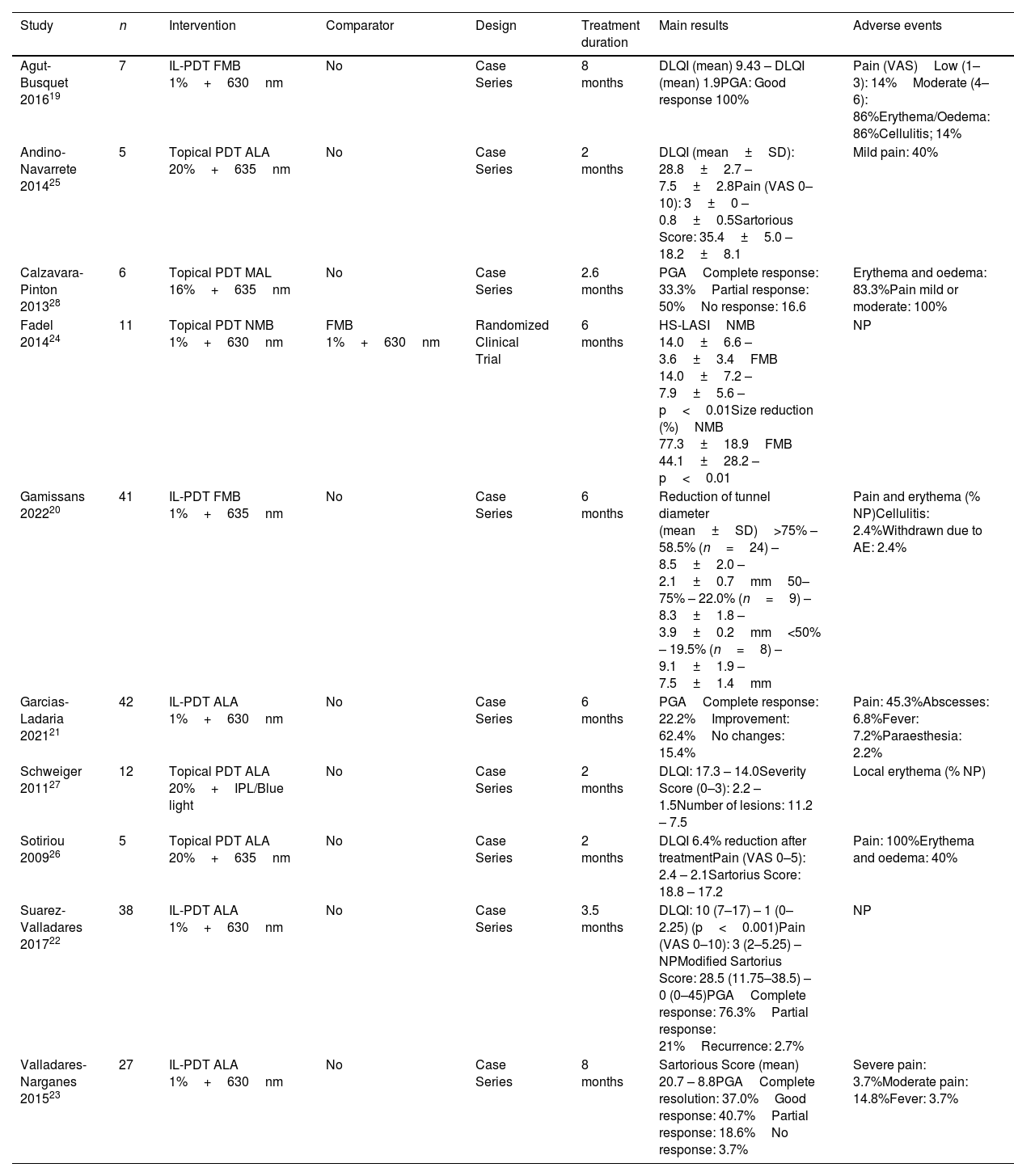
Summary of included studies assessing photodynamic therapy.
| Study | n | Intervention | Comparator | Design | Treatment duration | Main results | Adverse events |
|---|---|---|---|---|---|---|---|
| Agut-Busquet 201619 | 7 | IL-PDT FMB 1%+630nm | No | Case Series | 8 months | DLQI (mean) 9.43 – DLQI (mean) 1.9PGA: Good response 100% | Pain (VAS)Low (1–3): 14%Moderate (4–6): 86%Erythema/Oedema: 86%Cellulitis; 14% |
| Andino-Navarrete 201425 | 5 | Topical PDT ALA 20%+635nm | No | Case Series | 2 months | DLQI (mean±SD): 28.8±2.7 – 7.5±2.8Pain (VAS 0–10): 3±0 – 0.8±0.5Sartorious Score: 35.4±5.0 – 18.2±8.1 | Mild pain: 40% |
| Calzavara-Pinton 201328 | 6 | Topical PDT MAL 16%+635nm | No | Case Series | 2.6 months | PGAComplete response: 33.3%Partial response: 50%No response: 16.6 | Erythema and oedema: 83.3%Pain mild or moderate: 100% |
| Fadel 201424 | 11 | Topical PDT NMB 1%+630nm | FMB 1%+630nm | Randomized Clinical Trial | 6 months | HS-LASINMB 14.0±6.6 – 3.6±3.4FMB 14.0±7.2 – 7.9±5.6 – p<0.01Size reduction (%)NMB 77.3±18.9FMB 44.1±28.2 – p<0.01 | NP |
| Gamissans 202220 | 41 | IL-PDT FMB 1%+635nm | No | Case Series | 6 months | Reduction of tunnel diameter (mean±SD)>75% – 58.5% (n=24) – 8.5±2.0 – 2.1±0.7mm50–75% – 22.0% (n=9) – 8.3±1.8 – 3.9±0.2mm<50% – 19.5% (n=8) – 9.1±1.9 – 7.5±1.4mm | Pain and erythema (% NP)Cellulitis: 2.4%Withdrawn due to AE: 2.4% |
| Garcias-Ladaria 202121 | 42 | IL-PDT ALA 1%+630nm | No | Case Series | 6 months | PGAComplete response: 22.2%Improvement: 62.4%No changes: 15.4% | Pain: 45.3%Abscesses: 6.8%Fever: 7.2%Paraesthesia: 2.2% |
| Schweiger 201127 | 12 | Topical PDT ALA 20%+IPL/Blue light | No | Case Series | 2 months | DLQI: 17.3 – 14.0Severity Score (0–3): 2.2 – 1.5Number of lesions: 11.2 – 7.5 | Local erythema (% NP) |
| Sotiriou 200926 | 5 | Topical PDT ALA 20%+635nm | No | Case Series | 2 months | DLQI 6.4% reduction after treatmentPain (VAS 0–5): 2.4 – 2.1Sartorius Score: 18.8 – 17.2 | Pain: 100%Erythema and oedema: 40% |
| Suarez-Valladares 201722 | 38 | IL-PDT ALA 1%+630nm | No | Case Series | 3.5 months | DLQI: 10 (7–17) – 1 (0–2.25) (p<0.001)Pain (VAS 0–10): 3 (2–5.25) – NPModified Sartorius Score: 28.5 (11.75–38.5) – 0 (0–45)PGAComplete response: 76.3%Partial response: 21%Recurrence: 2.7% | NP |
| Valladares-Narganes 201523 | 27 | IL-PDT ALA 1%+630nm | No | Case Series | 8 months | Sartorious Score (mean) 20.7 – 8.8PGAComplete resolution: 37.0%Good response: 40.7%Partial response: 18.6%No response: 3.7% | Severe pain: 3.7%Moderate pain: 14.8%Fever: 3.7% |
IL: intralesional; PDT: photodynamic therapy; FMB: free methylene blue; DLQI: Dermatology Life Quality Index; PGA: physician global assessment; VAS: visual analogue scale; nm: nanometre; SD: standard deviation; NP: not provided; ALA: aminolevulinic acid; NMB: niosomal methylene blue; HS-LASI: HS lesion, area and severity index; IPL: intense pulsed light; MAL: methyl aminolevulinate.
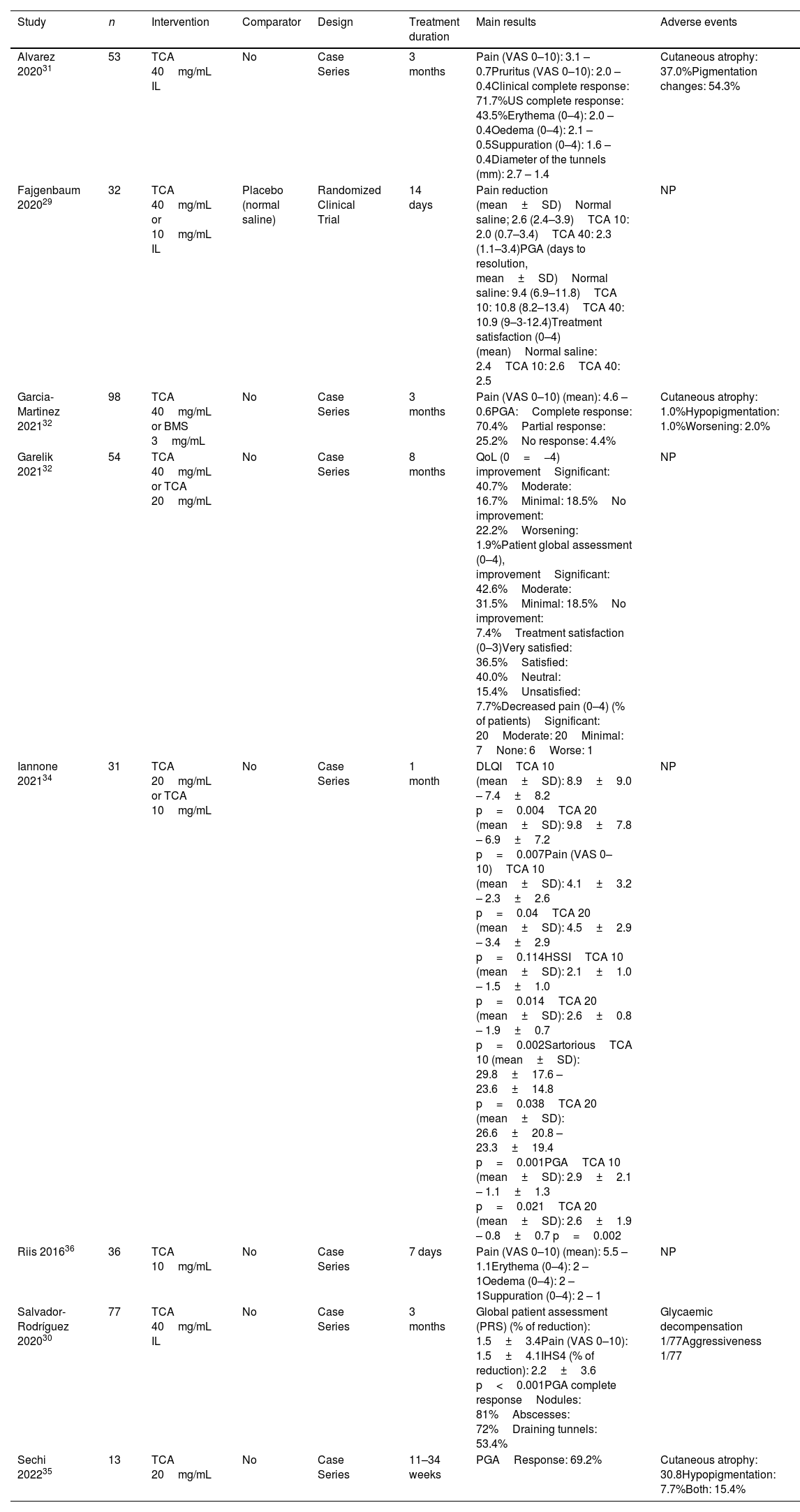
Summary of included studies assessing intralesional corticosteroids.
| Study | n | Intervention | Comparator | Design | Treatment duration | Main results | Adverse events |
|---|---|---|---|---|---|---|---|
| Alvarez 202031 | 53 | TCA 40mg/mL IL | No | Case Series | 3 months | Pain (VAS 0–10): 3.1 – 0.7Pruritus (VAS 0–10): 2.0 – 0.4Clinical complete response: 71.7%US complete response: 43.5%Erythema (0–4): 2.0 – 0.4Oedema (0–4): 2.1 – 0.5Suppuration (0–4): 1.6 – 0.4Diameter of the tunnels (mm): 2.7 – 1.4 | Cutaneous atrophy: 37.0%Pigmentation changes: 54.3% |
| Fajgenbaum 202029 | 32 | TCA 40mg/mL or 10mg/mL IL | Placebo (normal saline) | Randomized Clinical Trial | 14 days | Pain reduction (mean±SD)Normal saline; 2.6 (2.4–3.9)TCA 10: 2.0 (0.7–3.4)TCA 40: 2.3 (1.1–3.4)PGA (days to resolution, mean±SD)Normal saline: 9.4 (6.9–11.8)TCA 10: 10.8 (8.2–13.4)TCA 40: 10.9 (9–3-12.4)Treatment satisfaction (0–4) (mean)Normal saline: 2.4TCA 10: 2.6TCA 40: 2.5 | NP |
| Garcia-Martinez 202132 | 98 | TCA 40mg/mL or BMS 3mg/mL | No | Case Series | 3 months | Pain (VAS 0–10) (mean): 4.6 –0.6PGA:Complete response: 70.4%Partial response: 25.2%No response: 4.4% | Cutaneous atrophy: 1.0%Hypopigmentation: 1.0%Worsening: 2.0% |
| Garelik 202132 | 54 | TCA 40mg/mL or TCA 20mg/mL | No | Case Series | 8 months | QoL (0=−4) improvementSignificant: 40.7%Moderate: 16.7%Minimal: 18.5%No improvement: 22.2%Worsening: 1.9%Patient global assessment (0–4), improvementSignificant: 42.6%Moderate: 31.5%Minimal: 18.5%No improvement: 7.4%Treatment satisfaction (0–3)Very satisfied: 36.5%Satisfied: 40.0%Neutral: 15.4%Unsatisfied: 7.7%Decreased pain (0–4) (% of patients)Significant: 20Moderate: 20Minimal: 7None: 6Worse: 1 | NP |
| Iannone 202134 | 31 | TCA 20mg/mL or TCA 10mg/mL | No | Case Series | 1 month | DLQITCA 10 (mean±SD): 8.9±9.0 – 7.4±8.2 p=0.004TCA 20 (mean±SD): 9.8±7.8 – 6.9±7.2 p=0.007Pain (VAS 0–10)TCA 10 (mean±SD): 4.1±3.2 – 2.3±2.6 p=0.04TCA 20 (mean±SD): 4.5±2.9 – 3.4±2.9 p=0.114HSSITCA 10 (mean±SD): 2.1±1.0 – 1.5±1.0 p=0.014TCA 20 (mean±SD): 2.6±0.8 – 1.9±0.7 p=0.002SartoriousTCA 10 (mean±SD): 29.8±17.6 – 23.6±14.8 p=0.038TCA 20 (mean±SD): 26.6±20.8 – 23.3±19.4 p=0.001PGATCA 10 (mean±SD): 2.9±2.1 – 1.1±1.3 p=0.021TCA 20 (mean±SD): 2.6±1.9 – 0.8±0.7 p=0.002 | NP |
| Riis 201636 | 36 | TCA 10mg/mL | No | Case Series | 7 days | Pain (VAS 0–10) (mean): 5.5 – 1.1Erythema (0–4): 2 – 1Oedema (0–4): 2 – 1Suppuration (0–4): 2 – 1 | NP |
| Salvador-Rodríguez 202030 | 77 | TCA 40mg/mL IL | No | Case Series | 3 months | Global patient assessment (PRS) (% of reduction): 1.5±3.4Pain (VAS 0–10): 1.5±4.1IHS4 (% of reduction): 2.2±3.6 p<0.001PGA complete responseNodules: 81%Abscesses: 72%Draining tunnels: 53.4% | Glycaemic decompensation 1/77Aggressiveness 1/77 |
| Sechi 202235 | 13 | TCA 20mg/mL | No | Case Series | 11–34 weeks | PGAResponse: 69.2% | Cutaneous atrophy: 30.8Hypopigmentation: 7.7%Both: 15.4% |
TCA: triamcinolone acetonide; IL: intralesional; SD: standard deviation; NP: not provided; PGA; physician global assessment; QoL: quality of life; IHS4: International Hidradenitis Suppurativa Severity Score System; US: ultrasound; BMS: betamethasone; HSSI: hidradenitis suppurativa severity index.
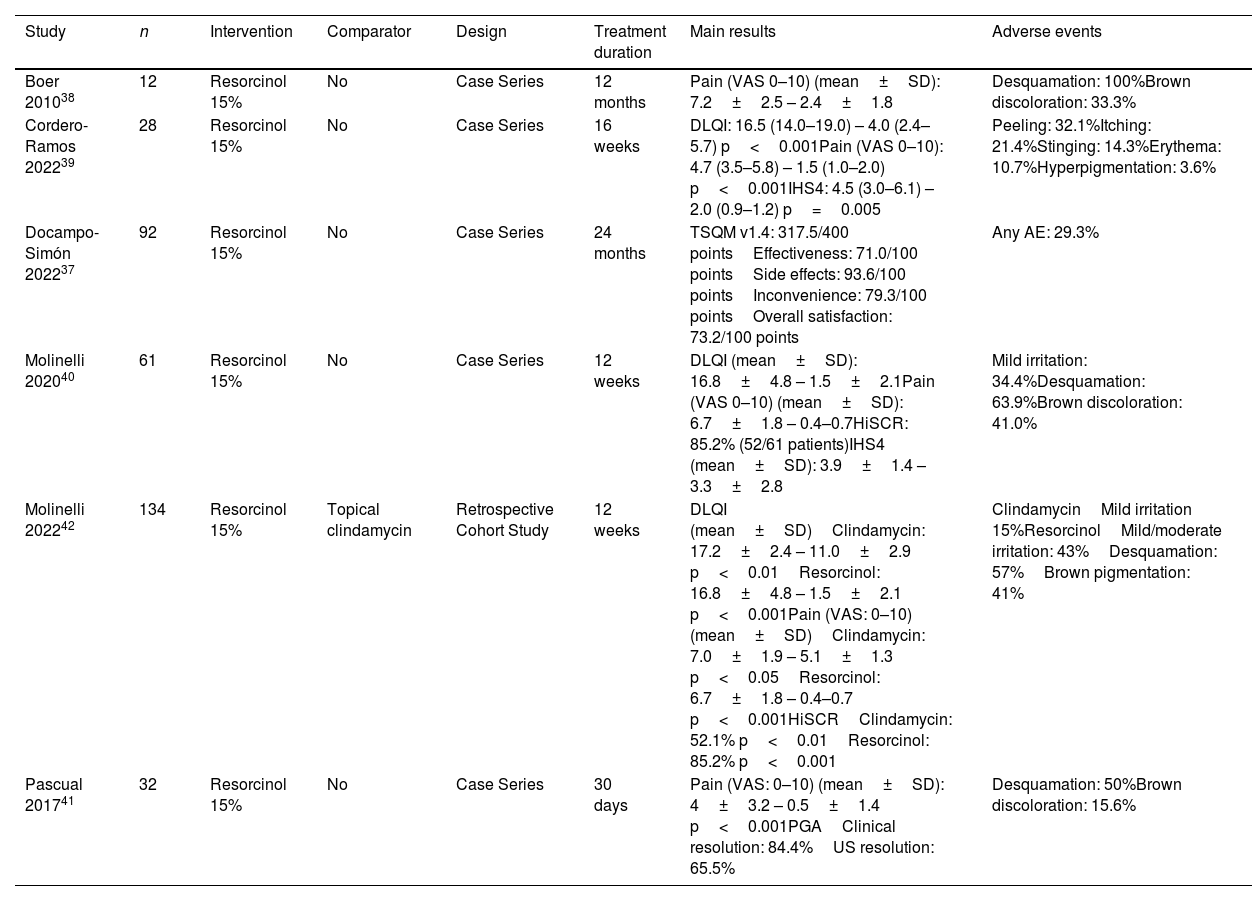
Summary of included studies assessing topical resorcinol.
| Study | n | Intervention | Comparator | Design | Treatment duration | Main results | Adverse events |
|---|---|---|---|---|---|---|---|
| Boer 201038 | 12 | Resorcinol 15% | No | Case Series | 12 months | Pain (VAS 0–10) (mean±SD): 7.2±2.5 – 2.4±1.8 | Desquamation: 100%Brown discoloration: 33.3% |
| Cordero-Ramos 202239 | 28 | Resorcinol 15% | No | Case Series | 16 weeks | DLQI: 16.5 (14.0–19.0) – 4.0 (2.4–5.7) p<0.001Pain (VAS 0–10): 4.7 (3.5–5.8) – 1.5 (1.0–2.0) p<0.001IHS4: 4.5 (3.0–6.1) – 2.0 (0.9–1.2) p=0.005 | Peeling: 32.1%Itching: 21.4%Stinging: 14.3%Erythema: 10.7%Hyperpigmentation: 3.6% |
| Docampo-Simón 202237 | 92 | Resorcinol 15% | No | Case Series | 24 months | TSQM v1.4: 317.5/400 pointsEffectiveness: 71.0/100 pointsSide effects: 93.6/100 pointsInconvenience: 79.3/100 pointsOverall satisfaction: 73.2/100 points | Any AE: 29.3% |
| Molinelli 202040 | 61 | Resorcinol 15% | No | Case Series | 12 weeks | DLQI (mean±SD): 16.8±4.8 – 1.5±2.1Pain (VAS 0–10) (mean±SD): 6.7±1.8 – 0.4–0.7HiSCR: 85.2% (52/61 patients)IHS4 (mean±SD): 3.9±1.4 – 3.3±2.8 | Mild irritation: 34.4%Desquamation: 63.9%Brown discoloration: 41.0% |
| Molinelli 202242 | 134 | Resorcinol 15% | Topical clindamycin | Retrospective Cohort Study | 12 weeks | DLQI (mean±SD)Clindamycin: 17.2±2.4 – 11.0±2.9 p<0.01Resorcinol: 16.8±4.8 – 1.5±2.1 p<0.001Pain (VAS: 0–10) (mean±SD)Clindamycin: 7.0±1.9 – 5.1±1.3 p<0.05Resorcinol: 6.7±1.8 – 0.4–0.7 p<0.001HiSCRClindamycin: 52.1% p<0.01Resorcinol: 85.2% p<0.001 | ClindamycinMild irritation 15%ResorcinolMild/moderate irritation: 43%Desquamation: 57%Brown pigmentation: 41% |
| Pascual 201741 | 32 | Resorcinol 15% | No | Case Series | 30 days | Pain (VAS: 0–10) (mean±SD): 4±3.2 – 0.5±1.4 p<0.001PGAClinical resolution: 84.4%US resolution: 65.5% | Desquamation: 50%Brown discoloration: 15.6% |
TSQM: treatment questionnaire for medication; VAS: visual analogue scale; SD: standard deviation; DLQI: Dermatology Life Quality Index; IHS4: International Hidradenitis Suppurativa Severity Score System; HiSCR: hidradenitis suppurativa clinical response; PGA: physician global assessment; US: ultrasound.
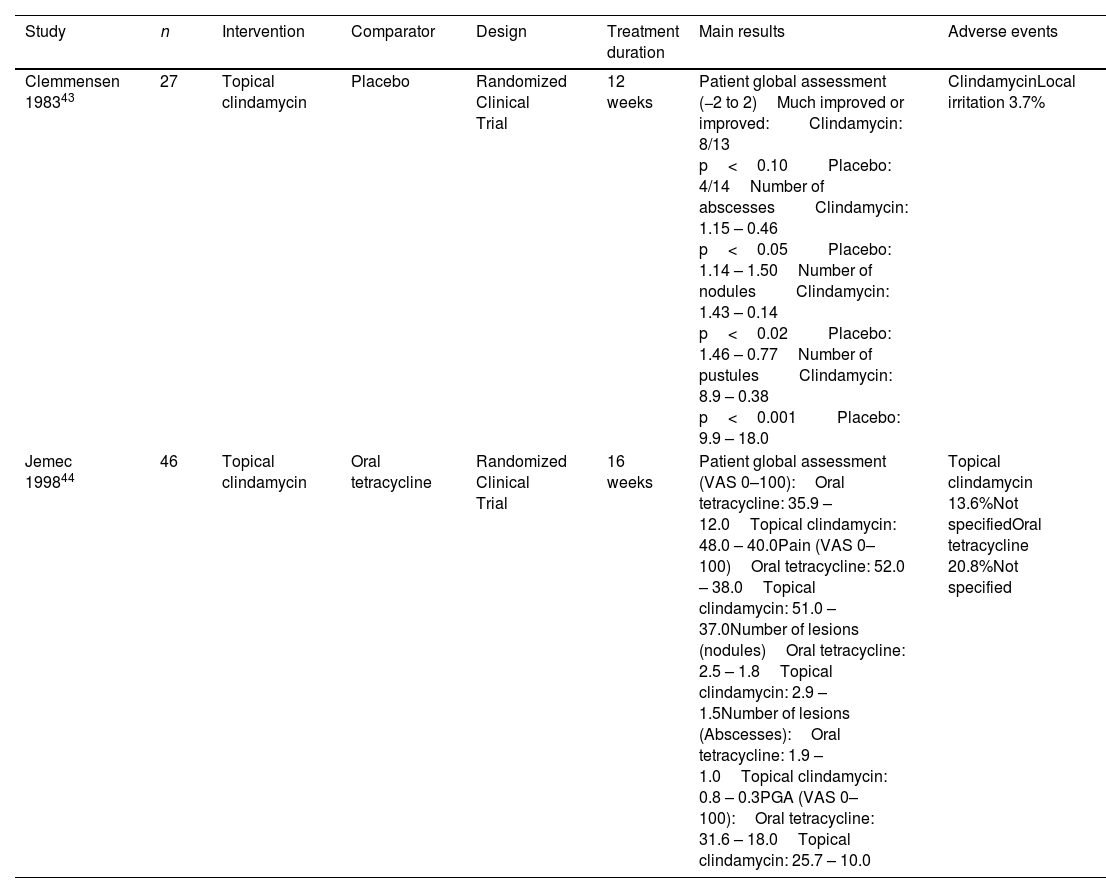
Summary of included studies assessing topical antibiotics.
| Study | n | Intervention | Comparator | Design | Treatment duration | Main results | Adverse events |
|---|---|---|---|---|---|---|---|
| Clemmensen 198343 | 27 | Topical clindamycin | Placebo | Randomized Clinical Trial | 12 weeks | Patient global assessment (−2 to 2)Much improved or improved:Clindamycin: 8/13 p<0.10Placebo: 4/14Number of abscessesClindamycin: 1.15 – 0.46 p<0.05Placebo: 1.14 – 1.50Number of nodulesClindamycin: 1.43 – 0.14 p<0.02Placebo: 1.46 – 0.77Number of pustulesClindamycin: 8.9 – 0.38 p<0.001Placebo: 9.9 – 18.0 | ClindamycinLocal irritation 3.7% |
| Jemec 199844 | 46 | Topical clindamycin | Oral tetracycline | Randomized Clinical Trial | 16 weeks | Patient global assessment (VAS 0–100):Oral tetracycline: 35.9 – 12.0Topical clindamycin: 48.0 – 40.0Pain (VAS 0–100)Oral tetracycline: 52.0 – 38.0Topical clindamycin: 51.0 – 37.0Number of lesions (nodules)Oral tetracycline: 2.5 – 1.8Topical clindamycin: 2.9 – 1.5Number of lesions (Abscesses):Oral tetracycline: 1.9 – 1.0Topical clindamycin: 0.8 – 0.3PGA (VAS 0–100):Oral tetracycline: 31.6 – 18.0Topical clindamycin: 25.7 – 10.0 | Topical clindamycin 13.6%Not specifiedOral tetracycline 20.8%Not specified |
DLQI: Dermatology Life Quality Index; SD: standard deviation; VAS: visual analogue scale; HiSCR: hidradenitis suppurativa clinical response.
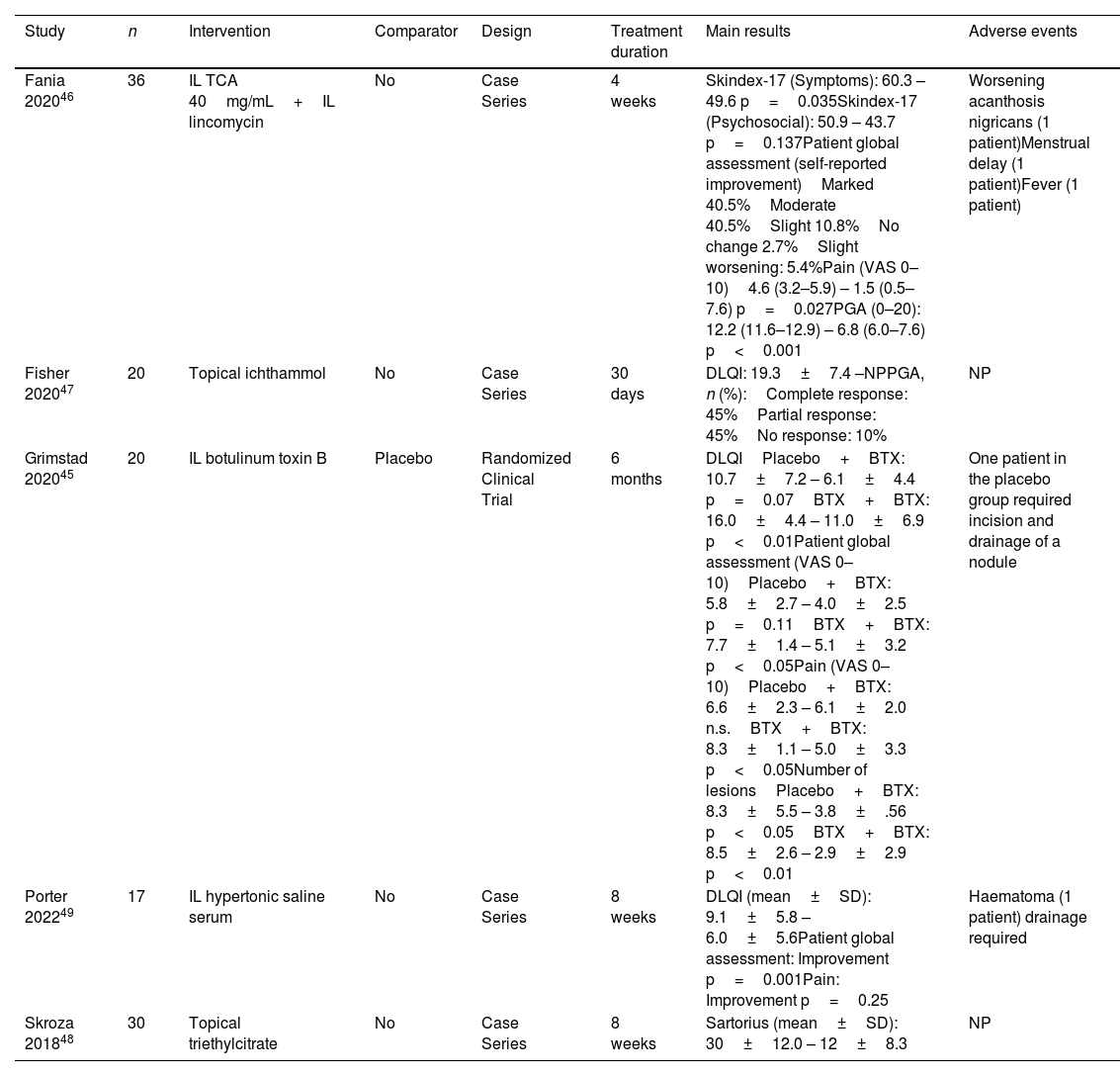
Summary of studies included with other interventions.
| Study | n | Intervention | Comparator | Design | Treatment duration | Main results | Adverse events |
|---|---|---|---|---|---|---|---|
| Fania 202046 | 36 | IL TCA 40mg/mL+IL lincomycin | No | Case Series | 4 weeks | Skindex-17 (Symptoms): 60.3 – 49.6 p=0.035Skindex-17 (Psychosocial): 50.9 – 43.7 p=0.137Patient global assessment (self-reported improvement)Marked 40.5%Moderate 40.5%Slight 10.8%No change 2.7%Slight worsening: 5.4%Pain (VAS 0–10)4.6 (3.2–5.9) – 1.5 (0.5–7.6) p=0.027PGA (0–20): 12.2 (11.6–12.9) – 6.8 (6.0–7.6) p<0.001 | Worsening acanthosis nigricans (1 patient)Menstrual delay (1 patient)Fever (1 patient) |
| Fisher 202047 | 20 | Topical ichthammol | No | Case Series | 30 days | DLQI: 19.3±7.4 –NPPGA, n (%):Complete response: 45%Partial response: 45%No response: 10% | NP |
| Grimstad 202045 | 20 | IL botulinum toxin B | Placebo | Randomized Clinical Trial | 6 months | DLQIPlacebo+BTX: 10.7±7.2 – 6.1±4.4 p=0.07BTX+BTX: 16.0±4.4 – 11.0±6.9 p<0.01Patient global assessment (VAS 0–10)Placebo+BTX: 5.8±2.7 – 4.0±2.5 p=0.11BTX+BTX: 7.7±1.4 – 5.1±3.2 p<0.05Pain (VAS 0–10)Placebo+BTX: 6.6±2.3 – 6.1±2.0 n.s.BTX+BTX: 8.3±1.1 – 5.0±3.3 p<0.05Number of lesionsPlacebo+BTX: 8.3±5.5 – 3.8±.56 p<0.05BTX+BTX: 8.5±2.6 – 2.9±2.9 p<0.01 | One patient in the placebo group required incision and drainage of a nodule |
| Porter 202249 | 17 | IL hypertonic saline serum | No | Case Series | 8 weeks | DLQI (mean±SD): 9.1±5.8 – 6.0±5.6Patient global assessment: Improvement p=0.001Pain: Improvement p=0.25 | Haematoma (1 patient) drainage required |
| Skroza 201848 | 30 | Topical triethylcitrate | No | Case Series | 8 weeks | Sartorius (mean±SD): 30±12.0 – 12±8.3 | NP |
IL: intralesional; DLQI: Dermatology Life Quality Index; BTX: botulinum toxin; VAS: visual analogue scale; TCA: triamcinolone acetonide; PGA: physician global assessment; NP: not provided.
Most papers reported on case series (n=25), with only five RCTs and a single cohort study. RCTs explored a variety of interventions: topical PDT-comparing a niosomal formulation of 1% methylene blue (NMB) to free methylene blue (FMB),24 IL corticosteroids-comparing triamcinolone acetonide (TCA) to normal saline,29 topical clindamycin-compared to either oral tetracycline44 or placebo,43 and botulinum toxin (BTX) vs placebo.45 The cohort study compared topical clindamycin to topical resorcinol.42 The remaining 25 case series evaluated the interventions, and others such as the combination of IL corticosteroids and lincomycin,46 topical ichthammol,47 topical triethyl citrate,48 or IL hypertonic saline.49
Considering the primary outcomes, 14 studies provided data on QoL, 12 of which used the DLQI.19,22,25–27,33,34,39,40,42,45–47,49 Meanwhile 23 reported the frequency of patients with, at least, one AEs.19–21,23,25–28,30–32,35,37–46,49 The remaining outcomes were assessed in a lower number of studies: patient global assessment of disease status (n=7)24,30,33,43–45,49, pain (n=20)22,23,25,26,29–34,36,38–42,44,45,49, pruritus31 (n=1), disease activity (n=17),20,22–27,30,34,36,39,40,42,44,45,48,49 physician global assessment (n=16),19,21–23,28–32,34,35,41,44,46,47,49 and treatment satisfaction (n=4)29,33,37,46(SI, Table 5).
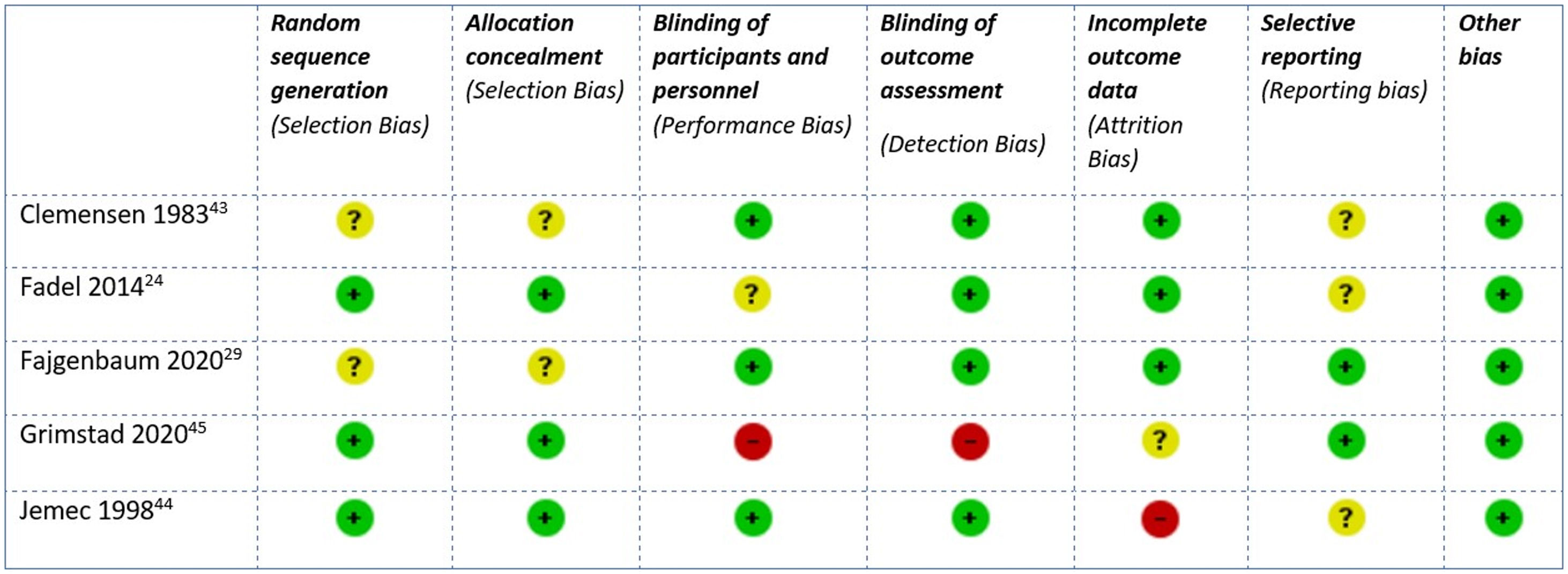
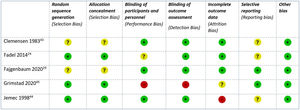
The RoB for the clinical trials included was considered low for three and high for two44,45 studies: the first with a high risk of attrition bias due to unbalanced withdrawals and a per-protocol analysis,44 the second with a high risk of performance and detection bias due to its open design45 (Fig. 2). The RoB of the only cohort study was also considered low.42 The remaining 25 case series were considered high risk by default.
Photodynamic therapyA total of 10 studies investigated the use of photodynamic (PDT), including 194 patients.19–28 These studies included five retrospective case series,19–21,28 five prospective case series,22,23,25–27 and one intraindividual split-body RCT.24 The type of lesions included was heterogeneous as nodules, abscesses and tunnels were considered. Most IL-PDT reports focus on the treatment of tunnels. Five studies explored the IL use of photosensitizer.19–23 Three different photosensitizers have been evaluated: methylene blue (MB), 5-aminolevulinic acid (ALA), and methyl aminolevulinate (MAL). Different sources and wavelengths have also been studied. Both the topical and IL-PDT provide similar data on efficacy, although topical application is associated with fewer AEs. The most frequently reported AE was pain during treatment, with up to 100% of the patients reporting pain in some studies. Ultrasound (US) was used to measure response in three case series.19,20,23
A single RCT with a low risk of bias assessed the efficacy profile of topical PDT with NMB compared to FMB.24 This study showed greater improvement in HS activity when a NMB was used (NMB, 75.9% vs FMB, 46.3% improvement). Additionally, it also showed a greater size reduction of the fistula. However, no QoL or safety data was provided.
IL corticosteroidsA total of eight studies have assessed treatment with corticosteroids for HS, including a total of 394 patients. All publications have focused on the use of IL corticosteroids29–36; no publications on topical corticosteroids were included in our SLR. A total of one RCT,29 four prospective30,31,34,36 and three retrospective case series32,33,35 were included. The studies include both acute lesions, such as nodules or abscesses, and more persistent, chronic lesions such as tunnels. TCA was the mostly frequently studied corticosteroid, with dosages ranging from 10mg/mL to 40mg/mL. AEs ranged from 2% to 56% of the cases in each study, with skin atrophy and hypopigmentation being the most frequent. US was used for treatment release or to determine response in five studies.30–32,34,35
A recent RCT assessed two IL doses of TCA (40mg/mL [TCA40] and 10mg/mL [TCA10]) vs placebo (normal saline).29 This RCT did not find any significant differences between any of the active treatment arms and placebo. Pain reduction 5 days after treatment assessed with a 0–10 visual analogue scale (VAS) was similar in all arms (TCA40 2.3 vs TCA10 2.0 vs placebo 2.6). Other outcomes such as the mean duration of the lesions until resolution and treatment satisfaction with were also similar between the groups.
Topical resorcinolSix publications have assessed the efficacy and safety profile of topical 15% resorcinol for HS, including a total of 359 patients.37–42 Three studies were prospective case series,39–41 two were retrospective case series,37,38 and one retrospective cohort study assessed the efficacy profile of 15% resorcinol vs topical clindamycin 1%. Resorcinol was superior to clindamycin in IHS4, HiSCR, pain, and DLQI improvement.42
No RCTs have ever assessed the efficacy or safety profile of topical resorcinol. Patients included showed a milder disease status (Hurley I and II), and the treated lesions were mostly nodules and abscesses. Resorcinol 15% was associated with improvement in DLQI, pain, HS activity, determined by IHS4 or HiSCR, and high treatment satisfaction. AEs occurred in 29–100% of the patients, with desquamation and reversible brown pigmentation being the most common sign. US was used in three publications to assess efficacy.39–41
AntibioticsTwo RCTs explored the use of topical antibiotics for HS including 73 patients.43,44 The first RCT, assessed the efficacy and safety profile of topical clindamycin 1% vs placebo.43 Patient assessment of symptoms was performed through a symptom diary, in which with every entry up to two points was added if patients noticed clinical improvement, and up to two points were subtracted in case of worsening. A statistically significant difference between topical clindamycin group and the placebo group (mean, +311 and −91, respectively) was reported. The number of inflammatory nodules, abscesses and pustules showed a significant reduction in patients on active treatment. Regarding AEs, only one episode of local irritation (3.7%) was reported. The second RCT compared topical 1% clindamycin to oral tetracyclines.44 When compared against oral tetracyclines, no significant difference was found between both treatments. Regarding safety, three AEs were described in the oral tetracycline group vs five in the topical clindamycin group, although no further description was provided.
Other interventionsRegarding other topical or IL interventions, a single RCT assessed the efficacy profile of a 3-month course of botulinum toxin type B(BTX) injections vs placebo, followed by 3 months of BTX in both groups.45 At 3 months, QoL improved by 6.6% in the BTX group (baseline DLQI BTX: 16- vs 3-month DLQI BTX: 9.9), and by 0.9% in the placebo group. Finally, four case series reported on the IL combination of TCA40 and lincomycin,46 topical 10% ichthammol,47 topical 1% triethyl citrate and ethyl linoleate G-peptide,48 or IL hypertonic saline.49 This last publication reported a significant improvement in DLQI and no. of lesions. All publications on antiseptic washes fulfilled the criteria to be included in this SLR.
DiscussionAs far as we know, this is the first SLR ever conducted on topical and IL treatments for HS. Several systematic reviews in the past five years have evaluated the efficacy or safety profile of treatments for HS: Robert,62 Gracia Cazaña,63 and Cuenca-Barrales.64 The first focuses on systemic therapies and laser (including PDT), the second on light treatments, and therefore includes data on PDT, while the last one focus exclusively on IL therapies. Since this last SLR, three new studies on IL treatments have been published and collected in our SLR.34,35,49 A prior Cochrane review published by Ingram et al., 8 years ago evaluated all interventions available for HS10; following Cochrane recommendations, the authors exclusively included RCTs, the best design to provide data on therapeutical options. However, the results show scarce evidence on understudied therapies, such as topical and IL therapies, with only three publications43,44,65 being included. Data from the remaining 29 studies included in our SLR were not considered, including two recently published RCTs. Finally, we should mention that none of these reviews included studies on topical resorcinol nor BTX.
The studies included in our SLR had a high degree of heterogeneity regarding patient characteristics, type of lesions included, assessed outcomes, and the follow-up period. This stresses the need to reach consensus on the core outcome domains that must be assessed in studies of patients with HS.66 This has proven to be a successful path in other diseases such as rheumatoid arthritis establishing a set of core outcome domains through OMERACT, which have been universally applied in the studies conducted ever since.67 Additionally, US was used in 12 out of 31 studies included in this review, both as an aid to treatment delivery or to assess the efficacy profile.19,20,23,30–32,34,39–41,49 This finding reinforces the utility of US in the management of patients with HS. On the other hand, the natural history of HS complicates the assessment of the efficacy profile of topical treatments for nodules and abscesses. Von der Werth described that nodules and abscesses often resolve within 7–10 days even without treatment,68 highlighting the need for a comparator to establish efficacy. For example, all lesions in which resorcinol was used were nodules and abscesses. Nonetheless, certain outcomes such as patient satisfaction can indirectly support efficacy.37
The last decade has seen an increase in the treatment options for moderate and severe HS, with the approval of adalimumab by the EMA (European Medicines Agency), and several new drugs seeking approval. This increased effort from the pharmaceutical industry has focused on moderate or severe forms of HS, resulting in a greater number of high-quality scientific studies (RCTs) in these subtypes of patients.69–72 However, the evidence supporting the treatment of mild forms of the disease remains poor. Mild HS is often the steppingstone towards moderate or severe forms. Additionally, even mild forms of the disease have an impact on QoL.73 The use of topical and IL drugs, commonly used in clinical practice in mild forms is not in general supported by RCTs. This fact is highlighted by this SLR and calls for the development of RCTs to determine the efficacy and safety profile of such treatments, regardless of pharmaceutical interests. Even though AE seem to be associated with the use of topical and IL therapies, these are, overall, mild and do not require treatment discontinuation.
The results regarding PDT suggest that it might be an effective and well-tolerated procedure, being the main AE, pain. Regarding IL corticosteroids, although the study conducted by Fajgenbaum is methodologically well-designed, two limitations hamper its external validity, the small volume of corticosteroids used (0.1mL) and the small sample size. Publications on resorcinol might suggest good efficacy and safety profile, but they lack a control group. A single cohort study confirmed the superiority of resorcinol over topical clindamycin, highlighting a therapeutic alternative that could avoid the promotion of antibiotic resistance. Finally, a single RCT with BTX suggests efficacy; a possible mechanism of action might be its anhidrotic effect that could change the skin microbiota.
The guidelines from the British Association of Dermatologists on the management of patients with HS only includes two topical or IL treatments in its recommendations: topical clindamycin and IL corticosteroids, the latter, exclusively for acute lesions. Both treatments are classified as strong recommendations, the benefits outweighing the risks. The European guidelines on the management of HS suggests74 the use of topical resorcinol, adapalene, and azelaic acid. It also mentions the use of IL corticosteroids, and, as experimental treatments, BTX, and PDT. However, only topical clindamycin for mild forms is included in its final algorithm.8 The North American guidelines on the management of HS, recommends skin cleaners and keratolytics, topical clindamycin, and IL corticosteroids.11 As we can see, guidelines are heterogeneous in their recommendation of topical and IL therapies, many times based on expert preferences and not on evidence support.
This SLR is hampered by some limitations. Every precaution was taken to gather all information available, with a wide search across three databases. The studies included show a wide degree of heterogeneity with respect to intervention, population, study design, outcomes and scales, and data analysis. In fact, the heterogeneity of interventions, together with the lack of a comparator in most of the studies, has prevented the development of the pre-planned meta-analysis. Additionally, follow-up periods in many of these studies are short, without further insight into the prolonged administration of these therapies.
In conclusion, although there is an increasing interest and studies on HS, the quality of evidence supporting the use of individual topical, or IL in HS is very low, with conditional support towards topical clindamycin, PDT with NMB and BTX. Further adequately designed RCTs, with standardized outcomes and homogeneous patient populations and lesions are welcomed to support clinical practice decisions.
Conflicts of interestJCP declared to be a consultant for Abbvie, Novartis and UCB in the field of HS. RHQ, VSG, AVM, IB, and FS declared no conflicts of interest whatsoever.