Cutaneous chronic graft-vs-host disease (GVHD) is a common complication of hematopoietic stem cell transplantation. Phototherapy is a therapeutic option for patients with skin involvement and for those who require high doses of corticosteroids. We analyze the cases treated in our department and review the literature.
Material and methodsAll patients with GVHD treated with phototherapy in the dermatology department of Hospital Universitario y Politécnico la Fe in Valencia, Spain between March 2011 and October 2014 were identified. Data were gathered retrospectively.
ResultsThere were 16 patients: 10 treated with psoralen–UV-A and 6 with narrowband–UV-B. Complete response was achieved in 9 patients and partial response in 7; 2 patients with partial responses relapsed after treatment. Ten patients were able to decrease their dose of corticosteroids during treatment, and a further 3 decreased the number of other immunosuppressant drugs. No serious adverse effects occurred.
ConclusionsPhototherapy is a good therapeutic option for patients with chronic GVHD with extensive cutaneous involvement, as well as for those who fail to respond to topical treatment or who have become steroid-dependent. The main benefits are that, as the treatment targets the skin, it reduces corticosteroid requirements and has a good safety profile. Treatment must be individualized and, in our experience, both the initial dose and the maximum dose per session can be lower than for other diseases.
La enfermedad injerto contra huésped (EICH) crónica cutánea es una complicación frecuente tras un trasplante de progenitores hematopoyéticos. La fototerapia es una modalidad terapéutica para pacientes con afectación cutánea o para aquellos que precisan altas dosis de corticoesteroides (CE). El objetivo de este estudio es revisar los casos tratados en nuestro servicio y hacer una revisión de la literatura.
Material y métodosRecogida de datos de manera retrospectiva de todos los casos tratados desde marzo de 2011 a octubre de 2014 en el Servicio de Dermatología del Hospital Universitario y Politécnico la Fe de Valencia.
ResultadosRecogimos un total de 16 pacientes, 10 tratados con PUVA y 6 con UVB-BE. Nueve pacientes obtuvieron una respuesta completa y 7 una respuesta parcial, aunque 2 recidivaron tras el tratamiento. Diez pacientes pudieron disminuir la dosis de CE durante el tratamiento y 3 pudieron disminuir el número de otros inmunosupresores. No se presentaron efectos adversos graves.
ConclusionesLa fototerapia es una buena opción terapéutica para pacientes con EICH crónica con gran afectación cutánea, para aquellos que no responden al tratamiento tópico o para pacientes corticodependientes. Su mayor beneficio es el de ser un tratamiento dirigido a la piel que permite ahorrar CE y que presenta un buen perfil de seguridad. La pauta de tratamiento se realiza de manera individualizada y, según nuestra experiencia, con dosis iniciales y dosis máximas por sesión menores que para otras enfermedades.
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative treatment available for a range of hematological and nonhematological diseases. The main obstacle to more widespread application of this technique is the problem of recognition of receptor tissue by immunity inherited from the donor, causing graft versus host disease (GVHD). Apart from tumor progression, this complication is the most common cause of morbidity and mortality associated with HSCT. However, the antitumor efficacy and potency of the technique also depend on the graft versus tumor effect mediated by T-lymphocytes of the donor, eradicating tumor cells.1,2
GVHD is classified as acute or chronic and these forms have different pathophysiological mechanisms and clinical presentation.3,4 In both cases, mucocutaneous involvement is the most common. Chronic GVHD usually presents in the first 100 days after HSCT and can affect almost any organ, leading initially to an underlying allogeneic autoimmune inflammatory process that progressively accumulates residual fibrotic changes. The cutaneous manifestations can be very varied and affect skin, mucosa, and skin appendages. Cutaneous manifestations can be divided into 2 groups: sclerotic (lichen sclerosus et atrophicus, morphea, and fascitis) and nonsclerotic (lichen planus and poikiloderma).5
There is no standard treatment and each patient is considered individually according to concurrent disease, affected organs, and the specific protocols for each center.5 The first-line treatment is high-dose corticosteroids (CS) alone or in combination with other immunosuppressants.5 More recently, new drugs and interventions have been introduced such as anti-tumor necrosis factor agents, imatinib, rituximab, and extracorporeal photoapheresis.6 All these treatments have side effects and increase the risk of infections and secondary neoplasms.
Phototherapy can thus play an important role in the management of both acute and chronic cutaneous GVHD as it is a therapy that targets the skin and has few side effects.7 UV-A light (320-400nm) has usually been used in combination with oral psoralen (PUVA), UV-A1 (340-400nm), and UV-B, which can be either broadband (280-320nm) or narrowband (311-312nm).
Material and MethodsThe present article describes the experience at our center in the treatment of patients with chronic cutaneous GVHD, covering all the clinical forms of presentation and the different types of phototherapy used (PUVA and narrowband UV-B).
Data CollectionWe retrospectively reviewed all patients with chronic cutaneous GVHD treated with phototherapy from March 2011 to October 2014. The information collected included age, sex, phototype, diagnosis, type of GVHD, site, and ocular and mucosal involvement.
Phototherapy RegimenIn those patients with contraindications for PUVA, with superficial lesions (erythema, lichen planus, and poikiloderma), low phototypes, or ocular or hepatic involvement, we decided to use narrowband UV-B. Those patients with high phototypes or sclerodermiform lesions received PUVA. In all cases, a Waldmann UV 7002 cabin was used.
The phototherapy regimen (initial dose, dose increase, and overall cumulative dose) was tailored for each patient, taking into account phototype, potentially photosensitizing concomitant medication, and type and site of lesions.
No photosensitive drug was withdrawn except for voriconazole, which was replaced with another prophylactic antifungal.
Where possible, the CS and immunosuppressant dose was tapered during treatment with phototherapy.
The following treatment-related information was collected: type of phototherapy, initial dose, cumulative dose, maximum dose per session, number of sessions a week, total number of sessions, number of cycles, and adverse effects.
Assessment of ResponseWe defined complete response (CR) as resolution of cutaneous GVHD and partial response as decrease in the clinical stage or clinical or symptomatic improvement (as measured by overall patient assessment and a pruritus scale).
Likewise, we noted the dose of prednisone and the number of immunosuppressants before and after treatment to assess possible reductions in usage (Figure 1).
Statistical AnalysisDescriptive statistics were calculated for the continuous variables (mean [SD]) and categoric variables (percentage [SD]). The normal distribution of the variables was assessed using a test for normality (Kolmogorov-Smirnov). Comparisons between means were analyzed with the t test.
Statistical analysis was performed with the IBM SPSS Statistics program, version 18 (SPSS Inc, Chicago, USA). Statistical significance was set at P<.05.
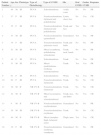
ResultsCharacteristics of PatientsThe main characteristics of the study population are summarized in Table 1. In total, 16 patients were included, 9 men (56%) and 7 women (44%), with a mean age of 48 (4) years. Twelve patients (75%) had phototype III and 4 (25%) had phototype II skin. Three patients (19%) showed sclerodermiform manifestations while 9 (56%) had nonsclerodermiform manifestations. The remaining 4 patients (25%) had mixed lesions. The lesions were located on the trunk in 5 patients (31%), limbs in 2 patients (12%), and multiple areas in 6 (38%). Three patients (19%) had generalized lesions. Five patients (31%) had oral GVHD and 7 (44%) had ocular disease.
Baseline Characteristics of the Study Population of Type of Response.
| Patient Number | Age, y | Sex | Phototype | Type of Phototherapy | Type of GVHD | Site | Oral GVHD | Ocular GVHD | Response |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | III | PUVA | Sclerodermiform | Axilla, waist | No | No | PR |
| 2 | 57 | F | III | PUVA | Nonslcerodermiform (lichenoid and poikiloderma) | Limbs, chest, face | No | Yes | CR |
| 3 | 55 | F | III | PUVA | Nonslcerodermiform (lichenoid and poikiloderma) | Trunk and face | Yes | No | CR |
| 4 | 53 | M | III | PUVA | Nonslcerodermiform (generalized) | Generalized | Yes | Yes | PR |
| 5 | 34 | F | III | PUVA | Nonsclerodermiform (pityriasis rosea) | Trunk and limbs | No | No | PR |
| 6 | 52 | M | III | PUVA | Mixed (exanthema, poikiloderma, scleroderma) | Trunk, neck, hands | No | No | PR |
| 7 | 48 | F | III | PUVA | Sclerodermiform | Trunk | No | Yes | PR |
| 8 | 51 | M | III | PUVA | Mixed (poikiloderma, erythema, sclerodermiform) | Trunk | Yes | Yes | PR |
| 9 | 61 | F | III | PUVA | Sclerodermiform | Wrists | Yes | Yes | PR |
| 10 | 62 | F | III | PUVA | Nonsclerodermiform (lichenoid) | Trunk | No | No | CR |
| 11 | 30 | M | II | NB UV-B | Nonsclerodermiform (lichenoid) | Trunk | No | No | CR |
| 12 | 52 | M | II | NB UV-B | Mixed (eczematous and sclerodermiform) | Trunk, face, and limbs | No | Yes | CR |
| 13 | 4 | M | II | NB UV-B | Nonslcerodermiform (erythroderma) | Generalized | No | No | PR |
| 14 | 58 | M | III | NB UV-B | Nonsclerodermiform (erythema) | Trunk | No | No | CR |
| 15 | 40 | M | II | NB UV-B | Mixed (morphea thigh, lichenoid arms) | Thigh, arms | Yes | Yes | CR |
| 16 | 39 | F | III | NB UV-B | Nonsclerodermiform (erythema, exanthema) | Generalized | No | No | PR |
Abbreviations: CR, complete response; F, female; GVHD, graft-versus-host disease; M, male; PR, partial response; PUVA, oral psoralen with UV-A; NB UV-B, narrowband UV-B.
Information on treatment is summarized in Table 2. Ten patients (63%) were treated with PUVA and 6 (37%) with narrowband UV-B. Fifteen patients (94%) followed a regimen with treatment on 3 days a week and 1 patient (6%) had treatment on 2 days a week. Fifteen patients (94%) received a single cycle of treatment, whereas 1 patient (6%) received 2 cycles. The mean number of sessions per cycle was 33 (4.6), with a mean duration per cycle of 3 months (range, 1-6 months). The mean starting dose with PUVA was 1.8 (0.18) J/cm2 while with narrowband UV-B it was 0.25 (0.03) J/cm2. The maximum dose used with PUVA was 4.4 (0.6) J/cm2 (range, 2-7J/cm2) while with narrowband UV-B it was 0.84 (0.16) J/cm2. The mean total cumulative dose per cycle with PUVA was 150 (38) J/cm2 while with narrowband UV-B it was 17 (4) J/cm2.
Phototherapy Regimen and Treatment Response.
| Sessions per week, n (%) | |
| 3 | 15 (94) |
| 2 | 1 (6) |
| Number of cycles, n (%) | |
| 2 | 15 (94) |
| 1 | 1 (6) |
| Sessions per Cycle | 33±4.6 |
| Cycle Duration, mo | 3 (1-6) |
| Starting dose, J/cm2 | |
| PUVA | 1,8±0.18 |
| NB UV-B | 0.25±0.03 |
| Maximum dose, J/cm2 | |
| PUVA | 4.4±0.6 |
| NB UV-B | 0.84±0.16 |
| Total cumulative dose, J/cm2 | |
| PUVA | 150±38 |
| NB UV-B | 17±4 |
| Complete response, n (%) | 9 (56) |
| Partial response, n (%) | 7 (44) |
| Decrease in prednisone dose, % | 24.9±5 |
Abbreviations: PUVA, oral psoralen with UV-A; NB UV-B, narrowband UV-B.
Information on treatment response is presented in Table 2. Nine patients (56%) achieved complete response and 7 (44%) achieved partial response. The reasons for treatment withdrawal were side effects (burns) in 2 patients (12%), underlying disease complications or GVHD in 3 (19%), and maximum response achieved in the remaining 11 patients (69%). All patients achieved favorable symptomatic response. All patients with oral GVHD showed a partial response and symptomatic improvement (related to swallowing ability).
Analysis of the effect of phototherapy treatment on CS dose showed a statistically significant decrease between the dose prior to and after phototherapy (32.5 [6.5] vs 25.5 [6]; P=.004). Ten patients (63%) were able to reduce CS dose during treatment, with a mean reduction in prednisone dose of 24.9% (5%). In addition, during treatment, 3 patients (19%) were able to reduce the number of immunosuppressive agents they were taking.
Adverse EffectsErythema was reported in 6 patients (38%). This event was mild in all cases except 2 (12%), in which the event led to discontinuation of treatment. In patients with mild erythema, the lesions resolved with emollients and topical CS; erythema did not recur after dose reduction and subsequent slower dose increases. The percentage of patients with erythema was similar in both types of treatment. Only 1 patient (6%) had pruritus during treatment.
Follow-upThe median duration of follow-up was 25 months (range, 2-42 months). During this period, 2 patients (12%) experienced worsening of their clinical symptoms after partial response. One of these was treated with another cycle of phototherapy and achieved partial response once again while the other required treatment with systemic drugs. All patients maintained immunosuppressants before, after, and during phototherapy.
DiscussionThe present study is, to the best of our knowledge, the first Spanish series published on the use of phototherapy for the treatment of patients with cutaneous GVHD. The main findings are as follows: a) treatment with phototherapy in cutaneous GVHD is safe; b) all patients showed clinical improvement with treatment; and c) phototherapy enabled a reduction in the CS dose administered.
Phototherapy may be a good therapeutic option for patients with chronic cutaneous GVHD who require systemic treatment to control their skin manifestations. The treatment targets the skin, and has few side effects. It has the advantage that it can enable CS sparing and reduction of the use of other immunosuppressants. The mechanism of action of phototherapy in GVHD is as yet unknown, but it appears that phenomena such as apoptosis, antiproliferative effects, and immunomodulatory effects (antigen presentation, cell activation, and cytokine release) may participate.8–10 It is still unclear whether phototherapy may have an impact on oral mucosa and other internal organs given its capacity to produce systemic immunomoduation.11,12
No randomized clinical trials have been conducted to compare phototherapy with other treatments. The evidence is based on case series that included a limited number of patients. Three studies have been published with at least 5 patients with chronic cutaneous GVHD treated with UV-A1.13–15 This type of phototherapy is not currently used in Spain. In these 3 published studies, doses of 50-60J/cm2 were used 3-5 times a week, with CR rates of 50%, although recurrences were common after stopping treatment. Five studies with at least 5 patients have also been published with oral or topical PUVA, with CR rates of 40% with both routes of administration.16–20 The authors reported some type of response in 70% of the patients and that treatment was more effective in the lichenoid forms. There are also 2 studies with UV-B (narrowband and broadband) in series of at least 5 patients,21,22 who were treated for 2-5 days a week, with symptomatic improvement and clearance of the lesions.
Our data show that phototherapy leads to clinical improvement in a high percentage of patients (100% in our series), also enabling a significant CS dose reduction (25%).
Given the lack of phototherapy protocols for a specific disease, psoriasis protocols are usually used, choosing the starting dose according to the minimum phototoxic dose, the minimum erythemogenic dose, and the phototype.23 Our patients were treated according to the psoriasis protocol, but we quickly realized that these patients displayed generalized erythema at lower doses. We therefore started treatment at lower doses than those corresponding to the skin phototype and escalated the dose with greater care, increasing 0.5J/cm2 every 2-3 sessions and performing a close follow-up of the patients (once weekly). In the case of PUVA, we found it difficult to increase the dose above 4J/cm2, as erythema occurred in most of our patients at this dose.
Given the special characteristics of these patients, they need tailored treatments, and it is not possible, in our experience, to apply rigid and fixed protocols. We can, however, point out the differences between phototherapy in patients with GVHD and phototherapy in other diseases12: lower starting doses are used, with slower dose escalation, more sessions, and lower cumulative doses. Improvement begins to become apparent from the tenth session, and the maximum response usually occurs after between 15 and 30 sessions.12 In addition, burns usually occur more easily (more erythema at lower doses), probably because of the disease itself and because many of the phototoxic agents cannot be discontinued or switched because they are essential for controlling the disease. One possible approach for setting the initial dose is to phototest the patients prior to starting treatment. This would probably not, however, avoid burns, which usually present during treatment and not during the first session.
Several considerations need to be taken into account before starting treatment. The immunosuppressive agents should not be withdrawn before, during, or after phototherapy. These are long-term treatments and often require the patient to attend the hospital, so it is necessary to take into account the distance from home and the dates of appointments with other specialists. Patients have several underlying diseases and will likely be taking multiple medications and possibly phototoxic drugs as well. After HSCT, patients often take voriconazole, an antifungal agent known to be an important phototoxic agent and photocarcinogen in immunosuppressed patients.24,25 When choosing between PUVA and UV-B, it is important to assess whether the patient has liver failure (due to GVHD or associated with drugs, infection, or other causes), ocular involvement (GVHD or cataracts), low phototypes, or a history of melanoma or nonmelanoma skin cancer. Narrowband UV-B is more comfortable for the patient and easier to manage for the dermatologist, and has fewer side effects and a lower long-term carcinogenic effect. Therefore, whenever possible, for superficial lesions, this should be the preferred type of phototherapy. For deeper lesions, as mentioned earlier, it will be necessary to use PUVA given that it penetrates further into the dermis and so is more effective.
Patients with GVHD are immunosuppressed patients who in their own right have a higher risk of melanoma and nonmelanoma skin cancer.26 This risk may be augmented by phototherapy even though the cumulative doses are lower. In any case, skin cancer should be ruled out before starting treatment, and follow-up is necessary. For patients with low phototypes and a history of skin cancer who are receiving immunosuppressive and/or phototoxic treatment (assessment in the event of withdrawal), the risk and benefit of phototherapy should be assessed individually.7
LimitationsThe present study has certain limitations. First, a limited number of patients are included, even though our center is a reference center for HSCT with many patients requiring management for GVHD. Nevertheless, we have collected data on our extensive experience in the management of these specific types of patients. Second, the clinical characteristics of the patients are heterogenous, probably because the disease is also very varied in its clinical manifestations. Therefore individually tailored treatment regimens need to be considered. Third, the study lacks a control group and so we are unable to draw definitive conclusions about CS and immunosuppressant sparring with phototherapy. Finally, the assessment of clinical response to treatment of the sclerodermiform lesions is complex given the difficulty of differentiating between active and inactive lesions.
The results are, therefore, difficult to interpret and compare with other therapies although, currently, these are the only information available given the lack of clinical trials.
ConclusionsPhototherapy may be a good therapeutic option for patients with extensive (lichenoid) or limited but deep (sclerodermiform) chronic cutaneous GVHD. The technique would also be valid for patients who do not respond to topical treatment or in patients in whom increased immunosuppressant doses would be associated with an increased risk of infections or interfere with the graft-versus-tumor response. The main advantage is that it is a targeted, safe, and effective treatment that enables sparing of CS doses.
To choose the best type of phototherapy (PUVA or narrowband UV-B) in each patient, we should bear in mind the following variables: phototype; type, extension, and depth of the lesions; possible involvement of other organs; and concurrent medication. The regimen will be individually tailored, generally with starting doses, intervals, and maximum and cumulative doses that are lower than for other diseases.
Ethical ResponsibilitiesProtection of human and animal subjects.The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data.The authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consent.The authors obtained the informed consent of patients and/or subjects mentioned in this article. The informed consent form is located in the archives of the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
I thank Pau for his tireless assistance and unconditional support.
Please cite this article as: Ballester-Sánchez R, Navarro-Mira MÁ, de Unamuno-Bustos B, Pujol-Marco C, Sanz-Caballer J, Botella-Estrada R. Análisis retrospectivo del papel de la fototerapia en la enfermedad injerto contra huésped crónica cutánea. Revisión de la literatura. Actas Dermosifiliogr. 2015;106:651–657.