In 1971, Owens and Freeman1 introduced the term perforating granuloma annulare (PGA) to describe a distinctive variant of granuloma annulare (GA) that affects approximately 5% of patients with this disorder.2 PGA is characterized by centrally umbilicated erythematous to violaceous papules, which often become crusted and leak clear or white content or evolve into ulcerations—either localized with a predilection for the extremities and dorsal aspect of the hands and fingers, or generalized.3 While the existence of papular umbilicated GA has been documented, it is likely that this sign indicates a variant of PGA. Although its underlying cause remains unknown, PGA, similar to granuloma annulare, has been associated with diabetes.2 Histologically, PGA is characterized by transepidermal elimination of necrobiotic collagen and granulomas in the dermis. No specific therapeutic approach has been established for PGA and its management to date has followed the broader protocols for GA.4
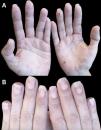
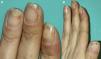
We report the case of a 43-year-old woman referred to our dermatology department for a rash on her hands. The lesions, described as painful, had appeared approximately 2½ years earlier during the first trimester of her only pregnancy, coinciding with the onset of nail involvement. Clinical history included active smoking and regular cannabis use. The patient did not engage in hobbies or activities requiring prolonged hand use and she denied undergoing manicure procedures. Furthermore, the patient was not on any prescribed drugs. Physical examination revealed the presence of violet, slightly hyperkeratotic, palpable and indurated papules on both palms, some with central depressions and crusts, along with residual non-palpable and hyperpigmented macules (Fig. 1). Remarkably, the patient exhibited tender, violaceous areas, distal onycholysis, lifting of the distal nail plate, with a white or yellowish appearance, along with subungual brownish-black punctate, hyperkeratosis and splinter hemorrhages in almost all nails (Figs. 1 and 2). Laboratory studies revealed mild hyperlipidemia and were negative for all parameters related to connective tissue disease, vasculitis, and infectious serologies, including syphilis. The microscopic description of a palm biopsy showed perivascular lymphocytes accompanied by interstitial histiocytic infiltration with mucin deposition, and areas of epidermal necrosis (Fig. 3). With a tentative diagnosis of localized PGA, topical corticosteroid treatment was started, yielding partial response. Oral hydroxychloroquine treatment was proposed but declined by the patient. Three years after the initial onset of the lesions, complete resolution was achieved with topical corticosteroids applied during the outbreaks. No treatment was applied to the nail lesions, and they followed a course parallel to the resolution of the hand lesions.
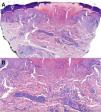
Panoramic microphotography stained with hematoxylin/eosin (H/E, 40×) from a biopsy obtained from a palmar lesion showing a disruption of the epidermis along with localized collagen alteration and an inflammatory infiltrate in the mid and upper dermis. A central keratotic plug was distinctly visible, imparting a cup-shaped contour to the lesion through which collagen was extruded transepidermally. Dermal perforation was evident (A). Histopathologic examination revealed collagen degeneration with necrobiotic features and a perivascular lymphohistiocytic infiltrate. Histiocytes arranged in a palisading pattern surrounded areas of degenerated collagen and mucin deposits (H/E, 200×) (B).
Due to its clinical characteristics, PGA can closely mimic several other conditions characterized by transepidermal extrusion of dermal content. PGA lesions exhibit clinical attributes, which are similar to those of other perforating dermatoses, such as cutaneous sarcoidosis and reactive perforating collagenosis. The location of the lesions may suggest palmoplantar punctate dermatoses, such as punctate palmoplantar keratoderma and punctate porokeratosis. The umbilicated and crusted appearance of primary PGA lesions can mimic certain skin neoplasms or infectious diseases, and the involvement of fingernails may even suggest inflammatory and rheumatologic dermatoses, such as vasculitis (Table 1). While cases of generalized PGA occurring during pregnancy have been documented5 and there is a described case discussing nail dystrophy in a psoriasiform presentation of perforating granuloma annulare,6 as far as we know, this is the first report in the literature of a case involving subungual PGA lesions, a sign that made diagnosis even more complex. Interestingly, literature does mention a significant relationship between GA and certain psychiatric disorders,7 including opioid dependency, and the association of GA with cigarette smoking,8 which was recorded in our patient.
Differential diagnoses considered for perforating granuloma annulare.
| Differential diagnoses for perforating granuloma annulare | ||||
|---|---|---|---|---|
| Infectious dermatoses | Perforating dermatoses | Palmoplantar punctate dermatoses | Inflammatory, rheumatologic and metabolic dermatoses | Onycholysis |
| Syphilis, rickettsiosis, arthropod bites, molluscum contagiosum, scabies, palmar warts, chickenpox, hand-foot-and-mouth disease, purpura fulminans, endocarditis, Haverhill fever, tuberculosis | Perforating granuloma annulare, sarcoidosis, reactive perforating collagenosis, perforating gout, foreign bodies, elastosis perforans serpiginosa, Kyrle's disease, mycosis fungoides, perforating cutaneous calcinosis | Punctate palmoplantar keratoderma, punctate porokeratosis, secondary keratoderma (neoplasms, toxins, drugs), spiny keratoderma, marginal papular acrokeratoderma, Flegel's disease | Small vessel vasculitis, psoriasis, cutaneous Crohn's disease, systemic sclerosis, rheumatoid arthritis (Bywaters’ lesion), dermatomyositis, porphyria cutanea tarda, erythropoietic protoporphyria, annular elastolytic giant cell granuloma | Psoriasis, trauma, exposure to alkalis or organic solvents, contact dermatitis to acrylates, drugs, pemphigus vulgaris, systemic lupus erythematosus, amyloidosis, multiple myeloma, pellagra, anemia, lung carcinoma |
In conclusion, PGA is a rare disorder whose diagnosis is challenging in which the clinical-pathological correlation is crucial to establish a definitive diagnosis. This article describes the first case report ever published of subungual PGA lesions.
Conflict of interestThe authors declare that they have no conflict of interest.