Spanish Autonomous Communities (ACs) are entitled to decide on the prescription requirements of their own territories, which can create inequalities in access to new drugs in the management of psoriasis. The objective of this study was to assess whether the level of restrictions in the access to new drugs for the management psoriasis was associated with the probability of achieving disease control measured using the Minimum Disease Activity (MDA) criteria. Therefore, we combined the results of 2 previous independent, cross-sectional studies: one that described the MDA in psoriasis by AC, and another that evaluated the level of restrictions to drug access by AC. We found that the higher the number of restrictions the lower the chances of achieving the MDA criteria (P=.013). Our results suggest that, in Spain, geographical differences in the access to new drugs may be creating health inequalities across the country.
Las comunidades autónomas (CC. AA.) españolas deciden sobre las condiciones de prescripción en su ámbito territorial, lo cual puede generar desigualdades en el acceso a nuevos fármacos. El objetivo del presente estudio fue evaluar la posible asociación entre el nivel de restricciones para acceder a nuevos fármacos en psoriasis y la probabilidad de alcanzar el control de la enfermedad psoriásica mediante la actividad mínima de la enfermedad (AME). Para ello se utilizaron los datos de dos estudios transversales independientes previos: uno que describió la AME en España, y otro que evaluó la cantidad de restricciones al acceso de fármacos por CC. AA. Tanto en el modelo crudo como en el ajustado se observó que, a mayor número de restricciones, menor fue el porcentaje de pacientes en AME (p=0,013). Los resultados obtenidos son compatibles con una situación de inequidad en salud en España que sería preciso abordar.
The development of biological drugs has represented a paradigm shift in the management of psoriasis, allowing for long-term disease control with a good safety profile1,2. However, their use can have a significant economic impact on the Spanish National Health System3 (NHS).
Because of the specific characteristics of the Spanish NHS, each autonomous community (AC) manages the health services of its territory and can decide on the conditions and requirements to prescribe drugs3,4. In the cross-sectional EQUIDAD study, a situation of disparities in the conditions of access to biological drugs for psoriasis among different ACs was observed, with diversity in criteria at both regional and local levels. The criteria did not appear to be based on decision-making methods that demonstrate the integration of scientific evidence5.
Independently, the Spanish Academy of Dermatology and Venereology Psoriasis Working Group (AEDV PWG) developed the minimal disease activity (MDA) variable for the management of psoriasis6, with the intent of using it as a measure of adequate disease management by characterizing patients with well-controlled psoriasis. After it was defined, a study was conducted at the end of 2022 to describe the situation of MDA, evaluating the percentage of psoriatic patients who meet the MDA criteria in a routine clinical practice in Spain (reference for MDA study).
The objective of the present study was to investigate the association between the probabilities of achieving MDA and the restrictions observed in access to new drugs in the treatment of psoriasis at both regional and local levels, combining data from the independent EQUIDAD and MDA trials of the AEDV.
Materials and methodsData sourceData from 2 independent cross-sectional studies were combined: the EQUIDAD5 study conducted by the AEDV, and the MDA (reference) study conducted by the AEDV PWG. Briefly, data for the EQUIDAD study were collected through an online questionnaire in March 2023, which evaluated the existing regional and local prescription conditions in each AC from an ecological perspective. The EQUIDAD study asked 2 dermatologists with management duties in each AC and only included restrictions reflected in official documents. Data for the MDA study were collected from July through November 2022 via consecutive sampling of patients from psoriasis units from various geographic areas covering almost all provinces of Spain.
Drugs evaluated in the studyNew therapeutic targets for biological drugs for the treatment psoriasis were included, for which funding resolutions were issued in the therapeutic positioning index (TPI) from 2016 through 2022 (ixekizumab, brodalumab, guselkumab, tildrakizumab, risankizumab, and bimekizumab).
Variables of interestThe following variables were collected:
- a)
Compliance with MDA: MDA was defined as the absence of active arthritis plus 3 of the following 6 characteristics: itch ≤ 1/10; scaling ≤ 2/10; redness ≤ 2/10; visibility ≤ 2/10; body surface area affected ≤ 2%; Dermatology Life Quality Index (DLQI) scores ≤ 2; and absence of lesions in special locations6.
- b)
Conditions for prescription in each AC: For patients in each AC, the level of restrictions greater than those required by the TPI at both regional and local levels for the prescription of the evaluated drugs was categorically recorded.
- c)
Other variables: Other variables from the MDA study were collected for each patient, such as sex, age, use of biological drugs, and AC of the treating hospital.
Qualitative variables were expressed as distributions of relative and absolute frequencies. Quantitative variables were expressed as mean and standard deviation. The probability of meeting the MDA criteria based on the level of regional and local restrictions described for each AC was analyzed using a crude multilevel logistic regression model, along with yet another model adjusted for possible confounders such as age, sex, or current use of biological drugs. A total of 3 sensitivity analyses were performed by regrouping the “level of restrictions” variable into different categories, which eventually led to a dichotomous model (less restrictive vs more restrictive ACs). Statistical significance was considered for p-values <0.05. All analyses were performed with STATA® (Stata Corp. 2021. Stata Statistical Software: version 17).
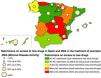
ResultsIn the MDA study, a final sample of 830 patients was obtained, although only 825 were considered for this work, representing a total of 17 different ACs (5 patients did not have their region on record). The mean age was 51.4 years (SD, 14.2) and 49.6% were on biological therapy. The MDA criteria were met in 40.8% of the patients included in the study. A total of 62.4% of patients are still being followed in 9 ACs without regional restrictions (although local restrictions may exist). Combining the MDA data with the levels of restrictions in the ACs based on the EQUIDAD study5, a trend can be seen, with 28.4% of patients with MDA in the 3 most restrictive ACs vs 50.7% of MDA in the 7 least restrictive ACs, with statistically significant differences (p <0.001) (fig. 1). The distribution of patients in the different AC groups based on the level of restrictions, as well as the characteristics of these groups are shown in Table 1.
General characteristics of the sample of patients included in the study and groups created after categorizing them based on the level of restrictions in their corresponding autonomous communities (ACs).
| Level of restrictions at AC and local level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No AC restrictions (local restrictions may exist though) | No AC restrictions (there are actual local restrictions) | A few AC restrictions (local restrictions may exist though) | Many AC restrictions (local restrictions may exist though) | Many AC restrictions (there are actual local restrictions) | ||||||
| N | Percentage | N | Percentage | N | Percentage | N | Percentage | N | Percentage | |
| Characteristics of the sample | ||||||||||
| No. of patients % | 365 | 44.2% | 150 | 18.2% | 49 | 5.9% | 145 | 17.6% | 116 | 14.1% |
| No. of ACs % | 7 41 | .2% | 2 | 11.8% | 1 | 5.9% | 4 | 23.5% | 3 | 17.6% |
| Sex | ||||||||||
| Male | 232 | 63.7% | 81 | 4.0% | 32 | 65.3% | 79 | 54.5% | 67 | 57.8% |
| Female | 132 | 36.3% | 69 | 6.0% | 17 | 34.7% | 66 | 45.5% | 49 | 42.2% |
| Use of biological therapies | ||||||||||
| No | 175 | 48.1% | 76 | 50.7% | 29 | 59.2% | 81 | 55.9% | 54 | 47.0% |
| Yes | 189 | 51.9% | 74 | 49.3% | 20 | 40.8% | 64 | 44.1% | 61 | 53.0% |
| Meets MDA | ||||||||||
| No | 180 | 49.3% | 94 | 62.7% | 33 | 67.3% | 99 | 68.3% | 83 | 71.6% |
| Yes | 185 | 50.7% | 56 | 37.3% | 16 | 32.7% | 46 | 31.7% | 33 | 28.4% |
| PASI <1 when completing the MDA questionnaire | ||||||||||
| No | 216 | 59.5% | 95 | 63.8% | 38 | 77.6% | 104 | 72.2% | 69 | 59.5% |
| Yes | 147 | 40.5% | 54 | 36.2% | 11 | 22.4% | 40 | 27.8% | 47 | 40.5% |
| Age (in years) mean (standard deviation) | 51 | (14.6) | 51 | (13.3) | 54 | (15.6) | 50 | (14.2) | 52 | (13.6) |
ACs, autonomous communities; MDA, minimal disease activity.
The level of regional restrictions considered was: “Many in AC”: regional restrictions greater than the therapeutic positioning index (TPI) for >50% of the study drugs. “Some in AC”: regional restrictions greater than the TPI existing in <50% of the study drugs. “No restrictions in AC”: no regional restrictions greater than the TPI for any drug.
In the crude multilevel logistic model (Supplementary data Table 1), this gradient of probability of achieving MDA was replicated at the same time the level of restrictions decreased. Being treated in an AC without regional restrictions (although local ones may exist) was independently associated with a higher probability of meeting the MDA criteria (OR, 2.39; 95%CI, 1.04-5.51). Although adjustment for the use of biological drugs (Supplementary data Table 1, adjusted model) showed a clear association with achieving MDA (OR, 5.86; 95%CI, 4.18-8.23), it did not eliminate the effect of geographical restrictions (OR, 2.64; 95%CI, 1.08-6.42). The model that categorized restrictions ordinally also showed a gradient of probability of achieving MDA as the level of restrictions dropped (OR, 1.27; 95%CI, 1.06-1.52; p=0.011).
As sensitivity analysis, the variable with the level of restrictions was regrouped into fewer categories, finding similar results in all cases (data not shown). The graphic representation of the percentage of MDA found in each AC and its level of restrictions is shown in Figure 1.
DiscussionThe presence of fewer restrictions, both local and regional, in access to new drugs for psoriasis seems to be associated with a higher probability of meeting the MDA criteria and, therefore, achieving adequate control of psoriatic disease. This association is independent of other factors, such as age or sex, and is maintained when including the use of biological drugs by the patient in the model.
The relationship found between controlling psoriatic disease and other factors such as the use of biological drugs or socio-economic level are factors already evaluated previously7,8. However, few studies have evaluated the impact of drug access-related restrictions on the control of psoriasis, focusing on comparisons across different countries9. The differences between ACs reported in this study are more relevant as they are differences within the same country where equitable health care for all patients is presumed.
It seems logical to think that the absence of specific restrictions could favor the prescription of drugs based on clinical needs, without being conditioned by financial or organizational factors. The fact that the relationship between restrictions and MDA is maintained when including the binary variable “biologicals” in the model raises the possibility that the ease of access to new drugs does not fully explain the better disease control in areas with fewer restrictions. Therefore, persistent differences could be due to reasons other than treatment with new drugs (which are also associated with the number of restrictions), or to the possibility of selecting specific new drugs that are more effective based on national or international recommendations2.
An alternative explanation is that access to a dermatologist may be easier in ACs with fewer restrictions, finding a higher proportion of patients with milder psoriasis who would more likely meet MDA criteria.
The strength of this study is that it is based on data from independent studies conducted before generating the hypothesis, which makes it unlikely that MDA measurements could be biased by the description of local restrictions. As for the limitations, the main one is that it is an ecological study, meaning that data on limitations are collected at AC level and the patients in whom MDA was measured may not be the same as those who experienced limitations in drug use. It is also possible that restrictions allow the total number of prescriptions in the AC to be higher and, although the benefit for each patient is smaller, the overall benefit for the population is higher. On the other hand, the use of consecutive sampling may not be equally representative of the population of psoriatic patients in all ACs. Additionally, evaluating restrictions from a regional perspective probably does not accurately reflect the specific restrictions existing at each hospital level.
In conclusion, the observed findings suggest that there is a relationship between a lower level of restrictions on access to biological drugs and a higher probability of adequate control of psoriatic disease. It would be desirable to homogenize the prescription criteria among the different Spanish ACs to promote greater equity in the treatment of psoriasis nationwide
Conflicts of interestManuel Sánchez Díaz has received funding for attending conferences from Sanofi, Lilly, Novartis, and Almirall.
Ángeles Flórez has received fees and/or support for educational activities and/or acted as a speaker and/or consultant and/or conducted clinical trials and/or research projects for Abbvie, ACELYRIN Inc, Alcedis GmbH, Almirall, Amgen, BMS, Celgene, Galderma, Incyte Corporation, Janssen-Cilag, Kyowa Kirin, Leo Pharma, Lilly, Novartis, Pfizer, Roche Farma, Sanofi-Aventis, Sun Pharma, Takeda, and UCB Biosciences GmbH.
Mercè Grau-Pérez has received funding for attending courses or conferences from Abbvie, Almirall, Janssen, Lilly, and Novartis.
Ignacio García-Doval has received funding for attending conferences from Abbvie, MSD, Pfizer, and Sanofi.
José-Manuel Carrascosa has received fees and/or support for educational activities and/or acted as a speaker and/or consultant and/or conducted clinical trials and/or research projects for Abbvie, Almirall, Amgen, BMS, Celgene, Galderma, Janssen-Cilag, Leo Pharma, Lilly, Novartis, Pfizer, Sanofi-Aventis, UCB, Sandoz, and Boehringer Ingelheim.
The MDA study has received financial support from Amirall and UCB laboratories. The laboratories did not participate in the design of the study, data analysis, obtaining results, or preparation of the publication manuscript.
National coordinator: Gregorio Carretero Hernández.
Regional coordinators: Mariano Ara Martín, Susana Armesto Alonso, Isabel Belinchón Romero, Noemi Eiris Salvado, Marta Ferrán Farrés, Cristina Galache Osuna, Marta García Bustínduy, Javier García Latasa de Araníbar, Marc Juliá Manresa, Mar Llamas Velasco, Anna López Ferrer, Mónica Roncero Riesco, Diana Ruiz Genao, Ricardo Ruiz-Villaverde, Laura Salgado Boquete, Caridad Soria Martínez, Eva Vilarrasa Rull. Collaborating Dermatologists: María Teresa Abalde Pintos, Alberto Alfaro Rubio, Gloria Aparicio Español, Beatriz Aranegui Arteaga, Ana Arechalde Pérez, Jorge Aróstegui Aguilar, Itrziar Arrue Michelena, Antoni Azón Masoliver, Cristina Bajo del Pozo, Adrián Ballano Ruiz, Ofelia Baniandrés Rodríguez, Susana Blanco Barios, María Teresa Bordel Gómez, José Manuel Carrascosa Carrillo, Ana María Carrizosa Esquivel, María Castellano González, Beatriz Castro Gutiérrez, María Antonia Concellón Doñate, Alberto Conde Taboada, Raquel Conejero del Mazo, Miriam Corral de la Calle, Raúl Corbalán Vélez, Neus Curcó Botargues, Sonia de la Fuente Meira, Cristina de las Heras, Francisco de la Torre Gomar, Elena del Alcázar Viladomiú, Rubén del Río Gil, Carmen Delgado Mucientes, Sonsoles Delgado Vicente, Miguel Duarte Ferrera, Carlos Durán Vián, Pilar Escalonilla García-Patos, José Manuel Fernández Armenteros, María Luisa Fernández Díaz, Cristina Fernández Sánchez, María Victoria Fuentelsaz del Barrio, Yolanda Fortuño Ruíz, José Frías Iniesta, Manuel Galán Gutiérrez, Fernando Gallardo Hernández, Mónica García Arpa, Carmen García Donoso, María José García Fernández de Villalta, Mercedes García Font, Miguel García Gil, Esther García Martínez, María García Sánchez, Fernando García Souto, Cristina Garrido Colmenero, Inma Gil Faure, María del Pino Gil Mateo, Daniel Godoy Díaz, Elena Godoy Gijón, Pilar Gómez Avivar, Pilar Gómez Centeno, Celia Gómez de Castro, Santiago Gómez Díez, Borja Gómez Vila, Álvaro González Cantero, Alicia González Quesada, Iris González Villanueva, Tamara Gracia Cazaña, Julia Paloma Hergueta Sánchez, Zaida Hernández Hernández, Carlos Hernández Montoya, Mercedes Hospital Gil, Marina Lacalle Calderón, Francisco Javier Laso Dosal, Victoria Lezcano Biosca, Angel López Ávila, Daniel López Castillo, María López-Escobar García-Prendes, José Luis López Estebaranz, Marta Lorda Espés, Dunia Luján Rodríguez, Laura Mahiques Santos, María Marcellán Fernández, Juan Márquez Henríquez, Laura Marqués Martín, Servando Eugenio Marrón Moya, Javier Martín Alcalde, Isabel Martín González, Esther Martín Sáez, Iñigo Martínez de Espronceda Esquerro, Ana Martínez de Salinas Quintana, Javier Mataix Martínez, Clara Matas Nadal, Almudena Mateu Puchades, Susana Medina Montalvo, Ana Menéndez García-Estrada, Julia Miralles Botella, Yosune Mitxelena Eceiza, Jordi Mollet Sánchez, Juan Monte Serrano, Andrea Montes Torres, Ana Morales Callaghan, Elisa Morales Larios, Ana Luisa Morales Moya, Fátima Moreno Suárez, Carlos Muñoz Santos, Nuria No Pérez, Jaume Notario Rosa, Nerea Ormaetxea Pérez, Laura Palacios García, José Pardo Sánchez, Silvia Pérez Barrio, José Antonio Pérez Caballero, Amalia Pérez Gil, Beatriz Pérez Suárez, María Pestana Eliche, María Pousa Martínez, Inés Poveda Montoyo, Lucía Prieto Torres, Laura Puebla Tornero, Lluis Puig Sanz, Josep Pujol Moncusí, Antonio Ramírez Andreo, José María Ramírez Concha, Aquilina Ramírez Santos, Leandra Reguero del Cura, Sheila Requena López, Miquel Ribera Pibernat, Josep Riera Monroig, Irene Río García, Raquel Rivera Díaz, Ignacio Rivera Fuentes, Vicenç Rocamora Durán, Sabela Rodríguez Blanco, Lourdes Rodríguez Freire, Fernando Rodríguez García, Marina Rodríguez Martín, Isabel Rodríguez Nevado, Guillermo Romero Aguilera, Alberto Romero Mate, Estrella Romero Sillero, José Carlos Ruiz Carrascosa, Javier Ruiz Martínez, Marc Sagristá García, Laura Saínz Gaspar, Ana Salas Martínez, Montserrat Salleras Redonet, Prado Sánchez Caminero, Elena Sánchez Largo, Javier Sánchez Pérez, María Pilar Sánchez Salas, Jorge Santos-Juanes Jiménez, Antonio Sahuquillo Torralba, Agustina Segurado Rodríguez, Miriam Sidró Sartos, Ruth Solanas Treviño, José Suárez Hernández, Rosa Taberner Ferrer, María Isabel Úbeda Clemente, Peru Urigoitia Ugalde, Francisco Vázquez López, Diana Velázquez Tarjuelo, David Vidal Sarro, Jaime Vilar Alejo, Isabel Villegas Romero, and Ignacio Yanguas Barona. The EQUIDAD study has been promoted and funded by the Fundación Piel Sana AEDV. Regional Coordinators of the ACs: Mariano Ara Martín, Salvador Arias Santiago, Rafael Botella Estrada, Javier Cañueto, Gregorio Carretero Hernández, Pablo Coto Segura, Esther de Eusebio Murillo, Ángeles Flórez, Francisco Javier García Latasa de Araníbar, Vicente García Patos Briones, Jesús Gardeazabal García, Cristina Gómez-Fernández, Sergio Hernández Ostiz, Rosa Izu Belloso, Ángel López Ávila, Pilar Manchado López, Ana Martín-Santiago, Iñigo Martinez De Espronceda Ezquerro, Almudena Mateu Puchades, Pedro Mercader García, Jaime Notario Rosa, Lucía Palacio Aller, Cristina Pérez Hortet, Lucía Quintana-Castanedo, Raquel Rivera, Vicenç Rocamora Durán, Isabel Mª Rodríguez Nevado, Ricardo Ruiz Villaverde, José Suárez, Lidia Trasobares Marugán, Carmen Vizán de Uña, Ignacio Yanguas, and Ander Zulaica-Garate.