Doxycycline is a drug widely used in dermatology. Its most frequent adverse effects are gastrointestinal discomfort and photosensitivity. Severe toxicity is unusual; only a few cases of epidermal necrolysis (EN), either toxic EN or Stevens-Johnson syndrome, are described in the literature. We describe a case of Stevens-Johnson syndrome in a teenager that occurred after the patient began doxycycline treatment for acne.
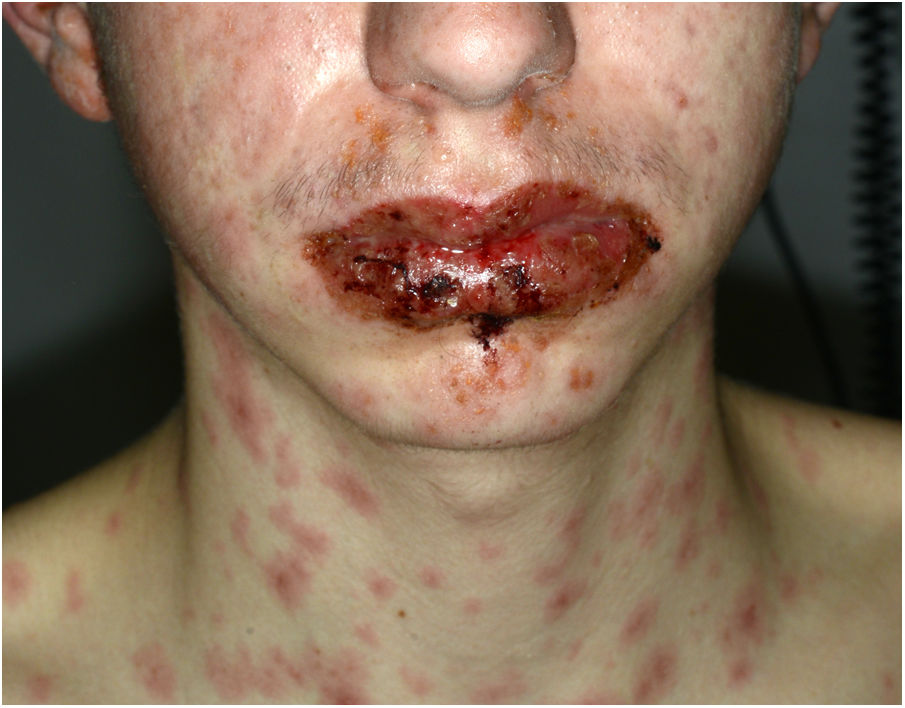
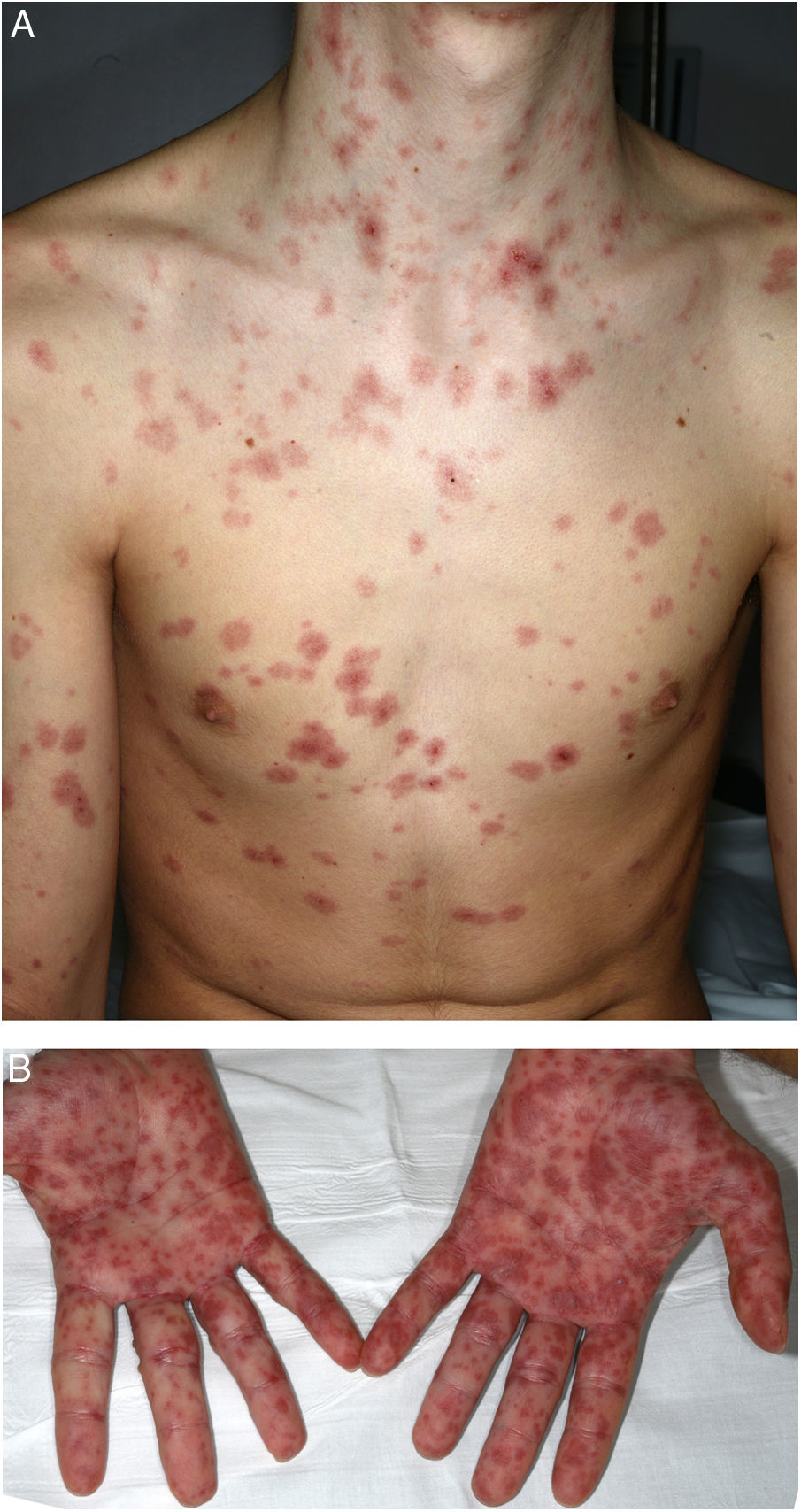
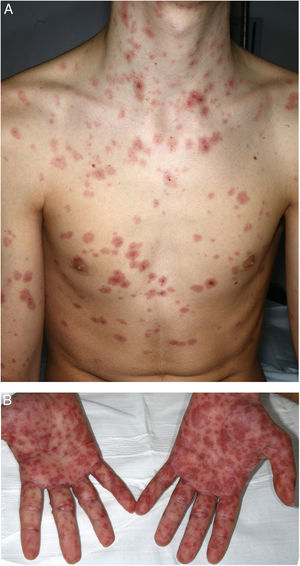
A 16-year-old boy with moderate papulopustular acne began treatment with topical adapalene and benzoyl peroxide and oral doxycycline (100 mg/d). Ten days after beginning antibiotic treatment he developed a low-grade fever and painful skin lesions on the hands and oral mucosa. He was initially diagnosed with mouth-hand-foot disease. Three days later the skin lesions spread to the entire body and oral involvement worsened, preventing ingestion of food or liquids. The patient then attended the emergency department of our hospital. General examination revealed that the patient was afebrile and normotensive, and showed marked signs of general compromise. He had edema of the lips with multiple erosions (Fig. 1) and whitish pseudomembranes that prevented opening of the mouth. Erythematous target lesions on the trunk, back, and extremities (Fig. 2A and B) covered more than 30% of the body surface, with detachment of less than 10% of the skin. Nikolsky sign was positive. Edema, erythema, and erosions were also observed on the glans and anal mucosa. An ophthalmological examination revealed no ocular alterations. The results of laboratory tests (complete blood count, blood glucose, creatinine, electrolyte balance, liver function tests) and chest radiography were normal. Polymerase chain reaction tests for herpes simplex virus 1, 2, 6, and 7, Epstein-Barr virus, cytomegalovirus, and Mycoplasma pneumoniae were negative. A skin biopsy showed multiple necrotic keratinocytes in the epidermis and perivascular lymphocytic infiltrate in the dermis. Based on the clinical and pathological features, the patient was diagnosed with Stevens-Johnson syndrome secondary to doxycycline treatment. Doxycycline treatment was discontinued and the patient was hospitalized and prescribed serum therapy, systemic corticosteroids (1 mg/kg/d) and topical 0.1% triamcinolone acetonide. Four days after hospitalization the mucocutaneous lesions had improved and the patient was able to eat. He was discharged after 7 days and continued corticosteroid treatment with a tapering dose. He remaining asymptomatic in subsequent medical check-ups.
EN is a severe hypersensitivity reaction with mucocutaneous detachment and high mortality, and is generally secondary to treatment with drugs such as allopurinol, anticonvulsants, anti-inflammatories, penicillins, or sulfa drugs (Table 1). In children, it is most commonly associated with sulfa drugs and anticonvulsants.1 Typically, the clinical picture develops between 4 and 28 days (most frequently 10–15 days) after starting treatment.2 Involvement of the oral, ocular, gastrointestinal, and genital mucosa is very common.1 The incidence of EN and the associated mortality appear to be lower in children than adults. In children, renal failure, bacterial infections, sepsis, epilepsy, and tumors are predictors of mortality. EN is associated with prolonged hospitalization and high healthcare costs.3 The most important aspect of EN treatment is discontinuation of treatment with the causal agent, admission to a specialist intensive care unit with experience in managing this condition, and provision of appropriate support measures.4 Treatment of EN is the subject of some controversy: corticosteroids, classical immunosuppressants, monoclonal anti-TNF-α agents, immunomodulators such as immunoglobulins, and plasmapheresis have all been used without clear evidence of effectiveness. A recent meta-analysis by Zimmermann et al5 reported an increase in survival in patients treated with cyclosporine, a slight benefit of corticosteroid treatment, and no response to immunoglobulin treatment. Several studies have questioned the efficacy of immunoglobulin treatment, which is not recommended in the 2016 UK guidelines for the management of EN.4 A recent Spanish study6 (n = 49) reported an increase in survival in patients treated with cyclosporine. Similar results were observed in retrospective studies conducted in Korea7 (n = 24) and India8 (n = 19) and published in the last year. However, a recent retrospective study with a large number of patients (n = 174; 95 treated with cyclosporine) observed no benefits in patients treated with this drug.9
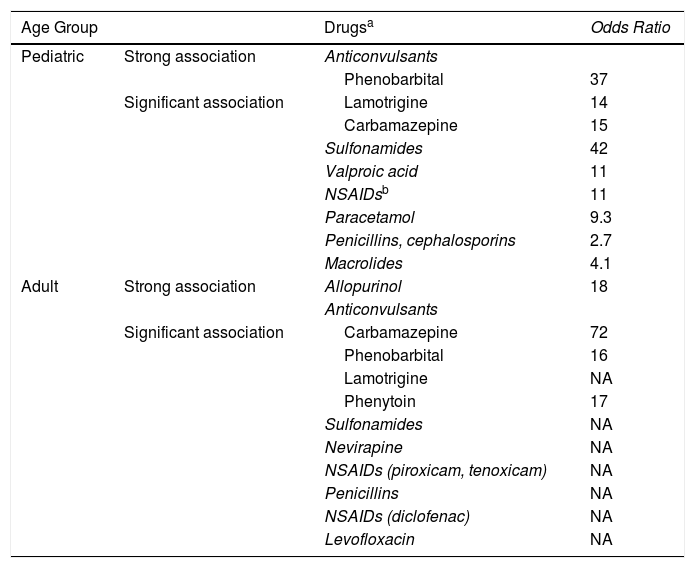
Main Drugs Implicated in Epidermal Necrolysis (Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome).
| Age Group | Drugsa | Odds Ratio | |
|---|---|---|---|
| Pediatric | Strong association | Anticonvulsants | |
| Phenobarbital | 37 | ||
| Significant association | Lamotrigine | 14 | |
| Carbamazepine | 15 | ||
| Sulfonamides | 42 | ||
| Valproic acid | 11 | ||
| NSAIDsb | 11 | ||
| Paracetamol | 9.3 | ||
| Penicillins, cephalosporins | 2.7 | ||
| Macrolides | 4.1 | ||
| Adult | Strong association | Allopurinol | 18 |
| Anticonvulsants | |||
| Significant association | Carbamazepine | 72 | |
| Phenobarbital | 16 | ||
| Lamotrigine | NA | ||
| Phenytoin | 17 | ||
| Sulfonamides | NA | ||
| Nevirapine | NA | ||
| NSAIDs (piroxicam, tenoxicam) | NA | ||
| Penicillins | NA | ||
| NSAIDs (diclofenac) | NA | ||
| Levofloxacin | NA |
Abbreviations: NSAID, nonsteroidal anti-inflammatory drugs; NA, not available.
a Between 80% and 100% of epidermal necrolysis cases are secondary to medication. More than 200 potentially responsible drugs have been described.
b At pediatric ages, the association between epidermal necrolysis and piroxicam or other NSAIDs of the oxicam group is rare.
Source: Ferrandiz-Pulido and Garcia-Patos,1 Levi et al,10 and Halevy et al.11
Doxycycline has antibiotic, anti-inflammatory, immunomodulatory, and collagenase properties. It has been used successfully to treat multiple dermatoses, including acne, rosacea, autoimmune bullous diseases, and neutrophilic diseases. Although contraindicated during pregnancy and in children under 8 years, it is a drug with a good safety profile. Serious adverse reactions such as EN are rare in patients treated with doxycycline.
The key to the management of patients with EN is immediate discontinuation of treatment with the causative drug, admission to a specialist intensive care unit, and provision of adequate support measures. It is important to rule out this condition in patients with mucosal compromise, even when the associated drug is not the most suspicious, as is the case with doxycycline.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morgado-Carrasco D, González Enseñat MA, Prat C, Vicente-Villa A. Síndrome de Stevens-Johnson secundario a tratamiento con doxiciclina en un adolescente. Actas Dermosifiliogr. 2020;111:615–617.