Hidradenitis suppurativa (HS) is a skin disease that significantly impacts patients’ quality of life. There are still uncertainties surrounding its epidemiology, natural history, and the safety and effectiveness of existing treatments. The Spanish Academy of Dermatology and Venereology has promoted the creation of a Spanish Registry of patients with HS (REHS). The aim of this article is to present the REHS and provide the initial results obtained.
MethodsThe REHS is a prospective, multicenter, observational study that collects the clinical and epidemiological characteristics of patients with HS, as well as the safety and effectiveness of the medical and surgical treatments received.
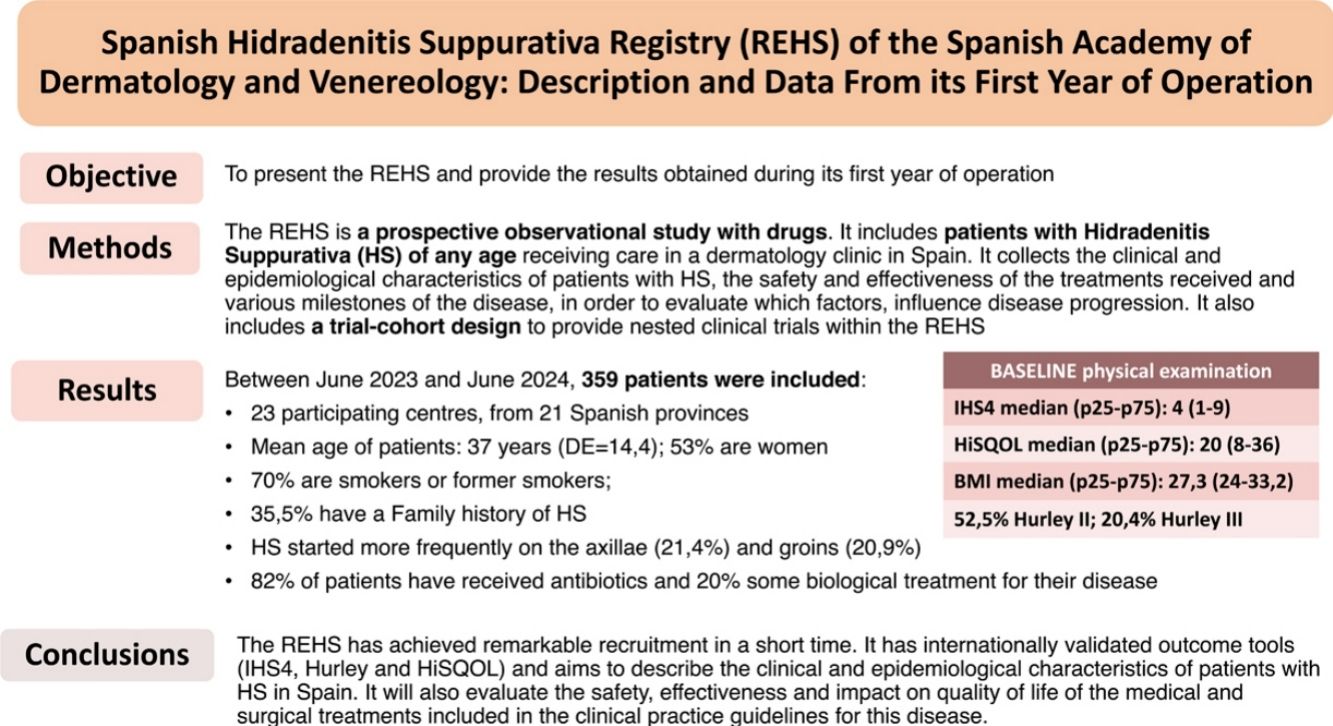
ResultsBetween June 2023 and June 2024, 359 patients were recruited from 23 Spanish centers. The average age of the patients is 37 years, and 53% of them are women. Over 70% of the patients are smokers or former smokers. One third have a family history of HS. The most frequent sites of disease onset are the axillae and groin. Median baseline IHS4 at recruitment was 4 (p25–p75=1–9), HiSQOL was 20 (p25–p75=8–36), and BMI was 27.3 (p25–p75=24–33.2). At least 82% of patients have received antibiotic therapy for their disease, and almost 20% a biologic drug.
ConclusionsWe present data from the first patients enrolled in the REHS, which will allow for the generation of evidence on the natural course of the disease, as well as the effectiveness and safety of treatments in HS.
La hidradenitis supurativa (HS) es una enfermedad que condiciona un gran impacto en la calidad de vida. Existen dudas sobre su epidemiología, historia natural y sobre la efectividad y la seguridad de los tratamientos existentes. La Academia Española de Dermatología y Venereología ha impulsado la creación de un Registro Español de pacientes con HS (REHS). El objetivo de este artículo es presentar el REHS y proporcionar los primeros resultados obtenidos.
MétodosEl REHS es un estudio observacional con medicamentos de seguimiento prospectivo, multicéntrico, que recoge las características clínicas y epidemiológicas de los pacientes con HS, así como la seguridad y la efectividad de los tratamientos médicos y quirúrgicos recibidos.
ResultadosEntre junio de 2023 y junio de 2024 se han recogido 359 pacientes de 23 centros españoles. La edad media de los pacientes es de 37 años, y un 53% son mujeres. Más del 70% de los pacientes son fumadores o exfumadores. Un tercio tiene antecedentes familiares de HS. Las localizaciones de inicio más frecuente de la enfermedad son axilas e ingles. La mediana de IHS4 basal al reclutamiento ha sido de 4 (p25-p75=1-9), la del HiSQOL de 20 (p25-p75=8-36) y el IMC de 27,3 (p25-p75=24-33,2). Al menos el 82% de los pacientes han recibido tratamientos antibióticos para su enfermedad, y casi un 20% un fármaco biológico.
ConclusionesPresentamos los datos de los primeros pacientes reclutados en el REHS, que permitirá generar evidencia sobre el curso natural de la enfermedad, así como la efectividad y la seguridad de los tratamientos en HS.
Hidradenitis suppurativa (HS) is a chronic skin disease characterized by the formation of inflammatory nodules, abscesses, and scars in areas such as the armpits, groin, and buttocks.1,2 It can have a profound impact on the patients’ quality of life, being one of the few dermatological diseases associated with an increased risk of suicide.3
Current data on HS indicate that its prevalence varies across regions, being more common in Western countries where it is estimated to be around 0.4–1.7%. This means that there could be more than 500,000 patients affected by this disease in Spain.4 Although a possible genetic predisposition for HS has been identified,5 as well as a close association with smoking and obesity, the pathogenesis of HS is still to be elucidated.6
Most cases of HS are mild and treated with topical or intralesional drugs,7 in addition to cycles of systemic antibiotics of variable duration. However, moderate and severe forms continue to represent a major therapeutic challenge, requiring the association of immunomodulatory drugs—systemic corticosteroids, biologics, etc.—and surgical procedures of variable extension.8 Although clinical practice guidelines recommend the use of multiple drugs,9 the available evidence on the safety and efficacy profile of these drugs in patients with HS, both in monotherapy and in combination, remains very limited and clinical results have proven unsatisfactory. It has been hypothesized that early therapeutic intervention could impact the course of the disease,10 but the available evidence in this regard is also still very limited. In addition, there are numerous topical treatment, educational and lifestyle modification measures whose efficacy has not been conveniently proven.2 Finally, surgical treatment of HS is essential at some points in the disease, with several modalities available.11 However, there is still insufficient evidence of which procedures are most effective and safe over time, nor of the optimal time to intervene, and the possible existence of a window of opportunity for surgical treatment has recently been raised.12
The Spanish Hidradenitis Suppurativa Registry (REHS) is a project promoted by the Spanish Academy of Dermatology and Venereology (AEDV) in collaboration with the Spanish Hidradenitis Suppurativa Working Group (GEHS), with the aim of providing clinical, epidemiological and care information on patients with HS in Spain, and to generate evidence to answer several of the questions that have been established as priorities at the international level on this disease. The objectives of this registry have been considered a priority by doctors and patients, occupying relevant positions in the James Lind Alliance's Top10 list of research priorities in HS13, and include: (a) To describe the short- and long-term safety and efficacy profile of systemic treatments for HS; (b) To evaluate if there are demographic, socioeconomic or clinical factors that can predict the prognosis of HS (which patients will progress, and which will not); (c) To assess if early diagnosis and treatment of the disease can impact the course of the disease and d) To assess the safety and efficacy profile of the different types of surgical procedures used in the treatment of HS.
The objective of this work is to describe the REHS and provide the first basic descriptive data of its first year of operation.
Material and methodsThe REHS is a prospective observational study with drugs. REHS is a multicenter prospective cohort, including patients with HS of any age who receive care in a dermatology clinic in Spain. It collects the sociodemographic and clinical characteristics of these patients, as well as the safety, effectiveness and impact on the quality of life of the treatments received. In relation to sociodemographic data, the registry includes information on the approximate address of the patient obtained from the CartoCiudad portal of the Spanish government,14 which will eventually allow us to extract their census section of residence and adjust for census data such as socioeconomic level. Information on the type of residence (urban/rural) will also be available, using spatial analysis techniques.15 In addition, the study offers patients the possibility of participating in clinical trials using a trial/cohort design,16 and was approved by Hospital Universitario y Pollitécnico La Fe Research Ethics Committee in March 2023 (FAE-HS-2023-01, Act No. 543).
Data are collected and managed using the electronic data capture tool Research Electronic Data Capture (REDCap).17 To participate in the REHS, participant centers must have, at least, 1 dermatologist with interest and responsibility in the supervision and treatment of patients with HS, an organization within the department that allows including all patients seen with this disease, and a minimum volume of 10 new patients per year who may be eligible to be included in the study. The inclusion criteria for patients are: patients with HS who are evaluated in a dermatology clinic in Spain, and diagnosis of HS by a dermatologist, according to the modified Dessau clinical criteria.18
The registry has (a) an inclusion visit, in which the patient's demographic and clinical data, history, a basic physical examination with data that allow for the automated calculation of IHS4 and the baseline HiSQOL quality of life questionnaire are collected, (b) a patient status form, which collects information on the dates of occurrence of various disease milestones during the patient's life, (c) a treatment form, which collects information on systemic treatments—which must be completed at recruitment and whenever the patient starts a new drug, including a field to report serious adverse events—and other surgical treatments; (d) follow-up visits, which are performed as scheduled in the routine clinical practice, including basic follow-up physical examinations with evaluation of IHS4 and the follow-up HiSQOL quality of life questionnaire. HiSQOL is the quality of life questionnaire developed by HISTORIC,19 the international alliance to establish outcome measures to improve the quality and consistency of studies evaluating treatments in HS. HiSQOL includes 17 items from 3 subdomains—symptoms, psychosocial and activity adaptations—collects information on the previous 7 days, and is the quality of life questionnaire currently recommended internationally in the management of HS.20
This work includes a few descriptive data on the patient's demographic and clinical characteristics at the time of inclusion, along with their past medical history. It also includes information on their situation at the first visit. These variables have been compiled into indices based on the nature of the variable (mean and standard deviation or median and p25–p75 for numerical variables depending on whether they fit a normal distribution or not, and frequencies and percentages for qualitative variables).
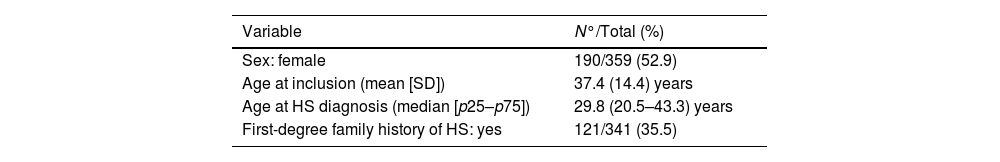
ResultsIn June 2024, the registry had a total of 359 patients from 23 hospitals across Spain, with representation from 21 provinces. Regarding the distribution by sex, 190 (52.9%) are women and 169 are men, with a mean age of 37.4 years (range, 11.7–74.3). Table 1 shows the patients’ clinical characteristics.
Demographic and clinical characteristics of patients in the Spanish Hidradenitis Suppurativa Registry during its first year of operation.
| Variable | N°/Total (%) |
|---|---|
| Sex: female | 190/359 (52.9) |
| Age at inclusion (mean [SD]) | 37.4 (14.4) years |
| Age at HS diagnosis (median [p25–p75]) | 29.8 (20.5–43.3) years |
| First-degree family history of HS: yes | 121/341 (35.5) |
| Anatomical location of first treated lesion, N=359 | N° (%) |
|---|---|
| Axillary | 77 (21.4) |
| Inguinal | 75 (20.9) |
| Sacral-gluteal | 47 (13.1) |
| Genital-perineal | 32 (8.9) |
| Head and neck | 19 (5.3) |
| Thighs | 6 (1.7) |
| Sub- or intermammary | 6 (1.7) |
| Abdomen | 1 (0.3) |
| Unknown | 96 (26.7) |
| Comorbidities | N°/Total (%) |
|---|---|
| HTN: Yes | 48/356 (13.5) |
| Diabetes: Yes | 42/356 (11.8) |
| Dyslipidemia: Yes | 49/356 (13.8) |
| Acne: Yes | 105/354 (29.7) |
| Anxiety: Yes | 91/351 (25.9) |
| Depression: Yes | 43/344 (12.5) |
| Insomnia: Yes | 33/342 (9.6) |
| Inflammatory bowel disease: Yes | 7/349 (2.0) |
| Arthritis: Yes | 17/355 (4.9) |
| Smoking status | |
| Current smoker | 201 (56.0) |
| Former smoker | 55 (15.3) |
| Never smoked | 99 (27.6) |
| Unknown | 4 (1.1) |
| Previous treatments | N°/Total (%) |
|---|---|
| Systemic antibiotics: Yes | 293/357 (82.1) |
| Intralesional/oral corticosteroids: Yes | 99/341 (29.0) |
| Oral retinoids: Yes | 56/340 (16.5) |
| Biologic drugs: Yes | 65/337 (19.3) |
| Number of biologics used (mean, SD) | 1.3 (0.6) |
| Surgical treatments: Yes | 193/338 (57.1) |
SD: standard deviation; HS: hidradenitis suppurativa; HTN: hypertension.
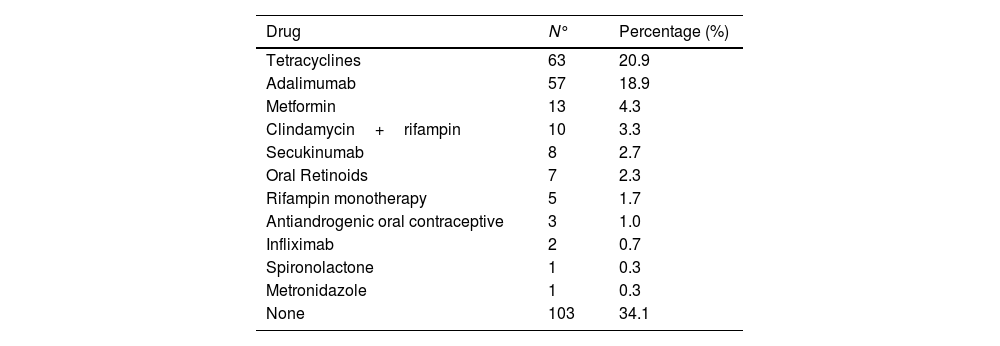
The most common anatomical locations that required some type of treatment in the first place were the armpits (21.4%) and the groin (20.9%). One third of the patients have first-degree family history of HS. More than half (56%) are smokers and 15.3% are former smokers. The most common comorbidities are acne (29%), anxiety (25.9%) and dyslipidemia (13.8%). Slightly more than half of the patients (53.8%) had required some surgical treatment for their HS prior to being included in the registry, 82.1% had used, at least, some antibiotic treatment, and almost a third had received systemic or intralesional corticosteroids (Table 2). A total of 25.9% were on antibiotics or had just started these at the time of the inclusion visit, while 22.3% were on biological treatment.
Active systemic treatments at inclusion visit (N=301).
| Drug | N° | Percentage (%) |
|---|---|---|
| Tetracyclines | 63 | 20.9 |
| Adalimumab | 57 | 18.9 |
| Metformin | 13 | 4.3 |
| Clindamycin+rifampin | 10 | 3.3 |
| Secukinumab | 8 | 2.7 |
| Oral Retinoids | 7 | 2.3 |
| Rifampin monotherapy | 5 | 1.7 |
| Antiandrogenic oral contraceptive | 3 | 1.0 |
| Infliximab | 2 | 0.7 |
| Spironolactone | 1 | 0.3 |
| Metronidazole | 1 | 0.3 |
| None | 103 | 34.1 |
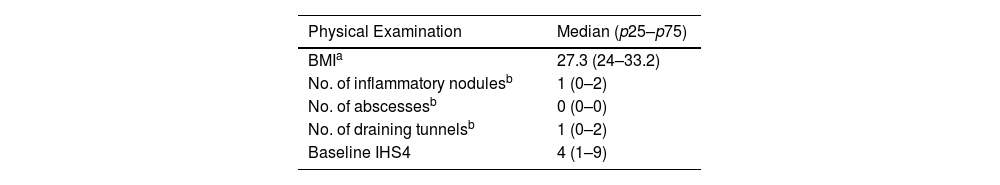
Table 3 shows data on the baseline physical examination. At the initial visit, the mean severity of HS measured by IHS4 was 7.8, with a median of 4 (p25–p75: 1–9). In 61.7% of patients, cutaneous ultrasound was used for the count of inflammatory nodules, abscesses and tunnels. Approximately half (52.5%) of the patients have Hurley II, and 20%, Hurley III. Regarding quality of life, the mean HiSQOL baseline score was 23.3 points.
Physical examination and quality of life at inclusion (N=359).
| Physical Examination | Median (p25–p75) |
|---|---|
| BMIa | 27.3 (24–33.2) |
| No. of inflammatory nodulesb | 1 (0–2) |
| No. of abscessesb | 0 (0–0) |
| No. of draining tunnelsb | 1 (0–2) |
| Baseline IHS4 | 4 (1–9) |
| N°/Total (%) | |
|---|---|
| Hurley stage | |
| I | 94/343 (27.4) |
| II | 179/343 (52.2) |
| III | 70/343 (20.4) |
| Phenotype | N°/Total (%) |
|---|---|
| Inflammatory | 207/335 (61.8) |
| Follicular | 76/335 (22.7) |
| Mixed | 52/335 (15.5) |
| Quality of life | Median (p25–p75) |
|---|---|
| Baseline HISQOL (0–68)c | 8–36) |
BMI: body mass index.
In its first year of operation, a total of 359 patients from 23 participant centers have been included in the REHS, with a wide age range (11–74 years), and a similar proportion of men and women. These data obtained in just one year are hopeful that the REHS can achieve a good representation of patients with HS who receive care in dermatology clinics in Spain.
Regarding clinical characteristics, the high rate of smokers and former smokers among REHS patients is concerning (overall, 71.3% vs 51.1% in the general Spanish population),21 since, in addition to smoking-related health complications, several studies have shown that smoking predisposes to the development of a more severe form of the disease too.5,9 In addition, the median BMI was 27.3 (overweight), and >10% of patients, despite being young, have hypertension and/or diabetes and/or dyslipidemia, meaning that the patients’ cardiovascular risk could be high.22
There are various initiatives for registries of HS patients,23 but very few prospective population studies similar to the REHS, and even fewer that have already come up with results.24–26 The Scandinavian registry HISREG started in 2012 and managed to recruit 225 patients within its first 3 years of operation,26 using the Hurley, Sartorius, NRS pain and DLQI scales, but not the IHS4 or HiSQOL, at the time it was developed. In 2019, they published their latest data up to the time of writing this article, in which they evaluated the treatments used within their first 3 years of operation (2013–2016),26 not having, at that time, any patients on biological treatment (adalimumab—the first biologic ever with an indication for HS—was authorized in 2016). Additionally, 2 other Italian registries have been conducted, the second (IRHIS 2) managing to recruit a total of 991 patients from 2015 to 2019. However, these were cross-sections without subsequent follow-up.27 Since 2015 there has been a European Registry for HS with the aim of better understanding the disease and its evolution, the association with comorbidities and to evaluate the efficacy of available treatments28; to date, the results of the Belgian section of this registry have been published, with 606 patients recruited in almost 10 years.29 In relation to physical examination scales, it is worth noting a similar distribution in the Hurley scale of REHS patients and those from the Belgian registry, a slightly higher percentage of stage III in the REHS (15% vs 20%), and a concordantly higher mean IHS4 in REHS patients in REHS patients (7.8; SD, 11.4) compared to the patients from the Belgian registry (5.2; SD, 12.3). This could be explained by a more severe involvement of REHS patients, or by the high rate of ultrasound use in the measurement of IHS4 detected within the REHS (in 61.7% of patients), which could have increased scale scores vs merely clinical observation,30 given that ultrasound allows more precise assessments and better identification of tunnels, which are the ones that score highest on the IHS4 scale. Of note, REHS patients have data which are similar to the Belgian registry in terms of family history of HS, smoking and mean recruitment age, as well as certain differences in terms of demographic data, with a greater proportion of women in the Belgian registry (67%), and certain comorbidities, with a greater proportion of depression in the Belgian registry (being its greatest comorbidity detected) with 43.8%, and of IBD, with 7.7%, vs 12.5% and 2% respectively in the REHS, being anxiety being more common among REHS patientes (25.9%), which was not recorded as a comorbidity in the Belgian registry.
The REHS has managed to include more than 350 patients in its first year, demonstrating a great recruitment capacity, and patients are in active follow-up. Other strengths of the REHS are: on the one hand, it includes a trial-cohort design, which will allow us to evaluate the efficacy of non-pharmacological interventions via I inter-group randomization within the registry. This design could be of particular interest to evaluate, for example, measures that demonstrate efficacy for smoking cessation, before scaling them nationwide to improve health outcomes in patients with HS. On the other hand, the REHS is currently the only registry of HS patients that has the combination of the validated outcome measures IHS4 and HiSQOL, so it will be able to provide relevant data in this regard and allow data comparisons from currently ongoing clinical trials. Of note, the mean HiSQOL score was 23.3 points (severe involvement), only 2 points lower than the mean HisQOL of patients included in phase 3 HS clinical trials.21 Finally, the REHS collects data on surgical procedures and will allow us to assess the effectiveness of combined medical and surgical treatments.
Recent publications suggest that early treatment in patients with HS could decrease HS-related complications.31 However, the evidence on the efficacy of these interventions is still very limited,32 and one of the main objectives of the REHS is precisely to evaluate if early diagnosis and treatment of HS can impact its course. A quarter of REHS patients at inclusion are on antibiotics and 22.3% on biological therapy. In the available data from the Scandinavian registry, no patient had ever been on biologics,26 and neither the Italian IRHIS 2 nor the Belgian one have provided any information on treatments to this date,27,29 which puts REHS in a favorable position to evaluate future responses to drugs both in monotherapy and combined, as well as eventually disease progression.
In conclusion, we present data from the first year of operation of the REHS. So far, we have patients from 23 different centers across Spain and more than 350 patients, with a growing rate of inclusion. The REHS has achieved remarkable recruitment in a short period of time, and has internationally validated outcome tools in physical examination and quality of life. It aspires to describe the clinical and epidemiological characteristics of patients with HS in Spain and will evaluate the safety, effectiveness and impact on the quality of life of medical and surgical treatments included in the clinical practice guidelines on the management of HS.
FundingThe REHS has been promoted by Fundación Piel Sana of the Spanish Academy of Dermatology and Venereology (AEDV) and receives funding from the pharmaceutical companies Novartis and UCB Pharma. None of these companies has been involved in the design or while the study was being conducted; in the collection, management, analysis or interpretation of data; in the preparation, review or approval of the manuscript; or in the decision to submit this manuscript for publication.
Conflicts of interestJ. Garcias-Ladaria has received help to attend congresses from Novartis and UCB, has been a speaker for Novartis and has participated in Advisory boards for Novartis.
J. Bassas Vila has received fees from Abbvie, Novartis and UCB to give conferences, subsidize his continuing education, participate in advisory boards and attend conferences and congresses.
L. Salgado-Boquete has collaborated with the following pharmaceutical companies as a researcher, advisor or speaker: Abbvie, Almirall, Amgen, Biogen, Boheringer, BMS, Celgene, Janssen-Cilag, Leo Pharma, Novartis, Sandoz, MSD-Schering-Plough, Lilly and UCB.
R. Rivera has participated in consultancies, as a speaker, as a researcher and has received help to attend congresses from Abbvie Laboratories, Boehringer, INCYTE, Johnson & Johnson, Novartis and UCB.
G. Martín-Ezquerra has received fees from Advisory of Novartis and UCB pharma.
E. Vilarrasa has received fees as a consultant/speaker and/or has participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Bayer, Biofrontera, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Galderma, Gebro, Isdin, Janssen, Leo-Pharma, Lilly, Merck-Serono, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi and UCB.
L.M. Pericet Fernández has collaborated as a speaker for Abbvie and Novartis.
I. Gracia-Darder has received fees as a speaker and/or travel expenses to attend meetings and/or has been involved in clinical trials sponsored by Almirall, Amgen, Cantabria Labs, Cumlaude lab, Isdin, Janssen, LEO Pharma, Lilly, Loreal, Novartis, Pfizer, Pierre Fabre, Sanofi, SVR and UCB.
V. Mora-Fernández has received fees as a speaker of talks, advisory board for his participation in clinical trials, as well as help for attendance at congresses and courses by AbbVie, Janssen, Novartis Pharma, Sanofi, LEO pharma, Isdin, Galderma and UCB Pharma.
M. Grau-Pérez has received travel and training assistance at scientific congresses from Abbvie, Janssen, Novartis, Pierre Fabre, Sanofi and UCB Pharma.
A. Martorell has received fees and/or travel grants and/or has acted as a member of advisory boards for Novartis, AbbVie, Janssen Cilag, UCB, Lilly, LEO Pharma, L’Oreal, Sanofi, Boehringer Ingelheim, Almirall, Bristol Myers Squib and Amgen. He has also worked as principal investigator in clinical trials funded by AbbVie, UCB, Jansen, Bristol Myers Squibb, Lilly, Galderma, Sanofi, Novartis and Legit Health.
We wish to thank all REHS researchers, for making an additional effort in clinical practice with data collection, as well as patients and/or their legal representatives for agreeing to their inclusion in the corresponding registry.
Jorge Arroyo (Hospital Universitario 12 de Octubre); Julia Verdaguer-Faja (Hospital Universitari Germans Trías i Pujol); Sandra Martínez Fernández (Complejo Hospitalario Universitario de Pontevedra); Manuel Sánchez-Díaz, Carlos Cuenca Barrales and Sofía Haselgruber (Hospital Universitario Virgen de las Nieves); Marta Ruano del Salado (Hospital Universitario de Torrejón); Begoña Udondo Gonzalez del Tanago (Hospital Universitario de Cruces); Alejandra Tomás and Francisco Javier García-Martinez (Clínica Universidad de Navarra); Antonio Sahuquillo Torralba and Rafael Botella (Hospital Universitario y Politécnico La Fe); Pedro Jose Navarro Guillamón (Hospital Universitario Virgen de la Victoria); Rubén Fuentes García (Hospital Universitari de la Santa Creu i Sant Pau); Irene Fuertes and Priscila Giavedoni (Hospital Clínic); Elena de Jesús García Verdú, Paola Merlo Gómez, Sonsoles María Aboín González and Lidia Trasobares (Hospital Universitario Príncipe de Asturias); María Elena Pelegrina Fernández, José González Rodríguez and Eduardo de la Rosa Fernández (Hospital Universitario Nuestra Señora de Candelaria); Paula Dávila Seijo (Hospital do Meixoeiro) and Ignacio García-Doval (AEDV Research Unit). The REHS has been developed within the Spanish Hidradenitis Suppurativa Working Group (GEHS).
The names of the members of the Spanish Registry of Hidradenitis Suppurativa (REHS) working group are listed in Appendix A.