The number of consultations for sexually transmitted infections (STIs) is increasing in Spain. The aim of this study was to describe and analyze the epidemiological, behavioral, clinical, and microbiological characteristics of patients registered at the STI unit of a tertiary hospital.
MethodsThis was a retrospective, single-center descriptive study carried out between 2010 and 2013 in a multidisciplinary unit specialized in STIs, situated in a tertiary hospital. Epidemiological, clinical, and behavioral data were gathered using a face-to-face interview and a standardized questionnaire. Samples were collected for microbiology analysis.
ResultsThe study included 546 patients: 96% were men, 41% had human immunodeficiency virus (HIV) infection, and 56% were men who have sex with men. The reasons for consultation were the following: urethritis; genital, anal, or perianal ulcers; proctitis; oral ulcers; sexual contact with a person with a known STI; and high-risk sexual contact. The most common microbiological diagnoses were Neisseria gonorrhoeae in urethritis, Treponema pallidum in genital and anal or perianal ulcers, and Chlamydia trachomatis lymphogranuloma venereum serovars in proctitis. The highest prevalences of the main STIs studied occurred in homosexual men with HIV infection.
ConclusionThis study confirms the increase in the incidence of STIs in recent years and the epidemiological characteristics of the HIV/STI epidemic in Spain.
Las infecciones de transmisión sexual son un motivo de consulta creciente en nuestro medio. El objetivo de este trabajo es describir y analizar las características epidemiológicas, conductuales, clínicas y microbiológicas de los pacientes registrados en una unidad de infecciones de transmisión sexual de un hospital terciario.
MétodosEstudio descriptivo, retrospectivo y unicéntrico realizado en una unidad multidisciplinar especializada en infecciones de transmisión sexual de un hospital terciario entre 2010 y 2013. Se recogieron datos epidemiológicos, clínicos y conductuales mediante entrevista oral abierta y cuestionario estandarizado, y se llevó a cabo la obtención de muestras para estudio microbiológico.
ResultadosSe estudiaron 546 pacientes, de los cuales fueron 96% varones, 41% infectados por el VIH, 56% hombres que tienen sexo con hombres. Los motivos de consulta más prevalentes fueron: uretritis, úlceras genitales y/o anales/perianales, proctitis, úlceras orales, contacto sexual de persona con ITS conocida y contacto sexual de riesgo. Los diagnósticos microbiológicos más frecuentes fueron: Neisseria gonorrhoeae en uretritis, Treponema pallidum en úlceras genitales y/o anales/perianales y Chlamydia trachomatis serovares de linfogranuloma venéreo en proctitis. Las principales ITS estudiadas fueron más prevalentes en varones homosexuales e infectados por el VIH.
ConclusiónSe confirma el incremento en la incidencia de las infecciones de transmisión sexual en los últimos años y las características epidemiológicas de la epidemia VIH/ITS de nuestro entorno.
The incidence of sexually transmitted infections (STIs) has risen in the past decade. According to the 2013 Report on the Epidemiological Sentinel Surveillance of STIs in Catalonia, published by the Catalan Center of Epidemiological Studies of STIs and AIDS (CEEISCAT), the annual incidence of syphilis increased from 1.4 to 10.6 cases per 100000 inhabitants between 2003 and 2013, while that of gonorrhea increased from 2.4 to 12.2 cases. The first cases of lymphogranuloma venereum (LGV) were reported in 2007, and the reported incidence in 2013 was 0.8 cases per 100000 inhabitants.1 The increase in STIs has been linked to numerous factors, such as sociocultural changes, socioeconomic factors, population explosion, migratory movements, and behavioral changes.2
STIs are an increasingly common reason for seeking care at primary health care centers and specialized outpatient and inpatient care units. To meet this demand, dedicated clinics, specialized in the management of STIs, have been created. These clinics are at the frontline of STI prevention.3 However, in view of the current epidemiological situation, the saturation of the health care system, and the complexity of certain cases of STIs, particularly in patients with concomitant human immunodeficiency virus (HIV) infection or other infections that require multidisciplinary management, the creation of specialized hospital-based STI units is justified. In 2008, our hospital opened a multidisciplinary unit for the management of acute STIs (urethritis, proctitis, genital and anal ulcers, and secondary syphilis), headed by the dermatology and infectious diseases departments, with participation of the microbiology and gynecology departments.
The aim of this study was to describe the epidemiological and clinical characteristics of the patients seen by this unit, based in a tertiary care hospital. We found no recent studies in the literature analyzing the current situation of STIs in high-risk patients in our setting.
Patients and MethodsThis was a descriptive, retrospective, single-center study in which we collected epidemiological, behavioral, clinical, and microbiological data for patients seen at the STI unit of Hospital Clínic de Barcelona (HCB) in Barcelona, Spain between January 2010 and December 2013.
The patients included patients who came to the unit spontaneously for an emergency visit or who had been sent from primary care centers affiliated with the hospital or from the hospital's dermatology, infectious disease, and emergency departments, among others.
Data pertaining to epidemiological factors, sexual behavior (type of relationship, number of partners, use of drugs before sexual activity, and others), and reasons for the visit were obtained by face-to-face interviews and completion of a standardized questionnaire used by the Catalan STI Registry (RITS).4 The RITS is a sentinel STI surveillance network for Catalonia that is coordinated by the CEEISCAT and Public Health Agency of Barcelona (ASPB). Use of the questionnaire was authorized by the institutional review board at HBC.
Our unit does not perform periodic monitoring of asymptomatic patients with risk behaviors that typically characterize STIs. It is an acute care unit, and monitoring all patients at risk for STIs and systematically studying all their contacts are not among our current objectives. Nonetheless, all patients are systematically informed of the need to tell their contacts to visit our unit or another center for evaluation and treatment.
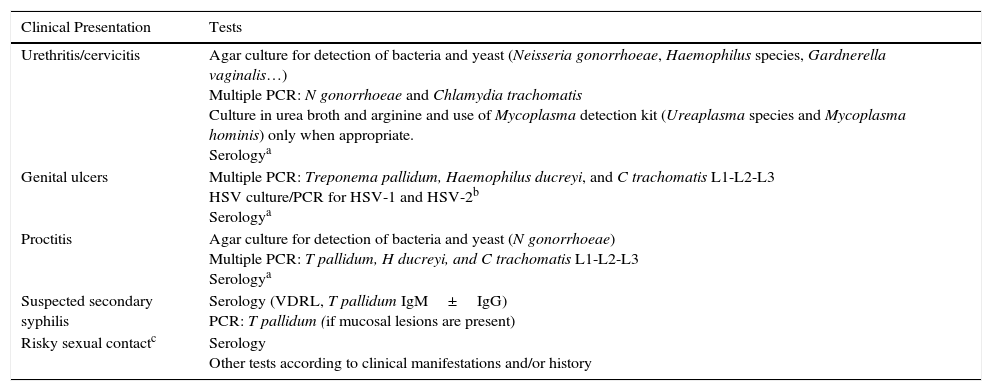
Samples were collected according to the protocol at our unit for testing and screening for STIs according to the clinical manifestations of the patient and/or his or her contacts (Table 1). The following microbiological tests were performed at the hospital's microbiology laboratory: a) culture for bacteria and yeast: urethral swabs were inoculated on chocolate agar, Thayer-Martin agar, and Sabouraud agar with chloramphenicol and gentamicin to test for Neisseria gonorrhoeae, Haemophilus species, and Candida species, vaginal and cervical swabs were inoculated on blood agar with colistin and nalidixic acid to test for Gardnerella vaginalis, and pharyngeal and rectal swabs were inoculated on selective media (chocolate agar and Thayer-Martin agar) to test for gonorrheal organisms; b) wet mount microscopy of the vagina to identify Trichomonas vaginalis; c) culture for Ureaplasma species and Mycoplasma hominis using the Mycoplasma IST2 kit (BioMérieux); d) MRC-5 cell culture for herpes simplex virus (HSV) and real-time polymerase chain reaction (PCR) for HSV-1 and HSV-2 (Nanogén); e) serology for syphilis, including VDRL (Spinreact) and enzyme immunoassay for Treponema pallidum-specific immunoglobulin (Ig) M and IgG antibodies(Trinity Biotech); f) detection of Chlamydia trachomatis antigen by immunochromatography (QuickVue Chlamydia Test, Quidel) up to June 2011 and thereafter with a real-time CT/NG assay (RT-PCT Anyplex CT/NG, Seegene); and g) PCR for T pallidum, Haemophilus ducreyi, LVG C trachomatis, HVS-1, and HSV-2 using STB-B40 ACE Detection (Seegene) up to May 2012 and after that with real-time PCR for T pallidum, H ducreyi, and LGV C trachomatis using RealCycler THLV (Progenie).
Test Protocol According to Clinical Presentation.
| Clinical Presentation | Tests |
|---|---|
| Urethritis/cervicitis | Agar culture for detection of bacteria and yeast (Neisseria gonorrhoeae, Haemophilus species, Gardnerella vaginalis…) Multiple PCR: N gonorrhoeae and Chlamydia trachomatis Culture in urea broth and arginine and use of Mycoplasma detection kit (Ureaplasma species and Mycoplasma hominis) only when appropriate. Serologya |
| Genital ulcers | Multiple PCR: Treponema pallidum, Haemophilus ducreyi, and C trachomatis L1-L2-L3 HSV culture/PCR for HSV-1 and HSV-2b Serologya |
| Proctitis | Agar culture for detection of bacteria and yeast (N gonorrhoeae) Multiple PCR: T pallidum, H ducreyi, and C trachomatis L1-L2-L3 Serologya |
| Suspected secondary syphilis | Serology (VDRL, T pallidum IgM±IgG) PCR: T pallidum (if mucosal lesions are present) |
| Risky sexual contactc | Serology Other tests according to clinical manifestations and/or history |
Abbreviations: HSV, herpes simplex virus; Ig, immunoglobulin; PCR, polymerase chain reaction.
We performed a descriptive analysis using measures of central tendency and dispersion for quantitative variables and distribution of frequencies for qualitative variables. We also performed a bivariate analysis with the χ2 or Fisher test, as appropriate. A 2-tailed P value of less than .05 was considered statistically significant.
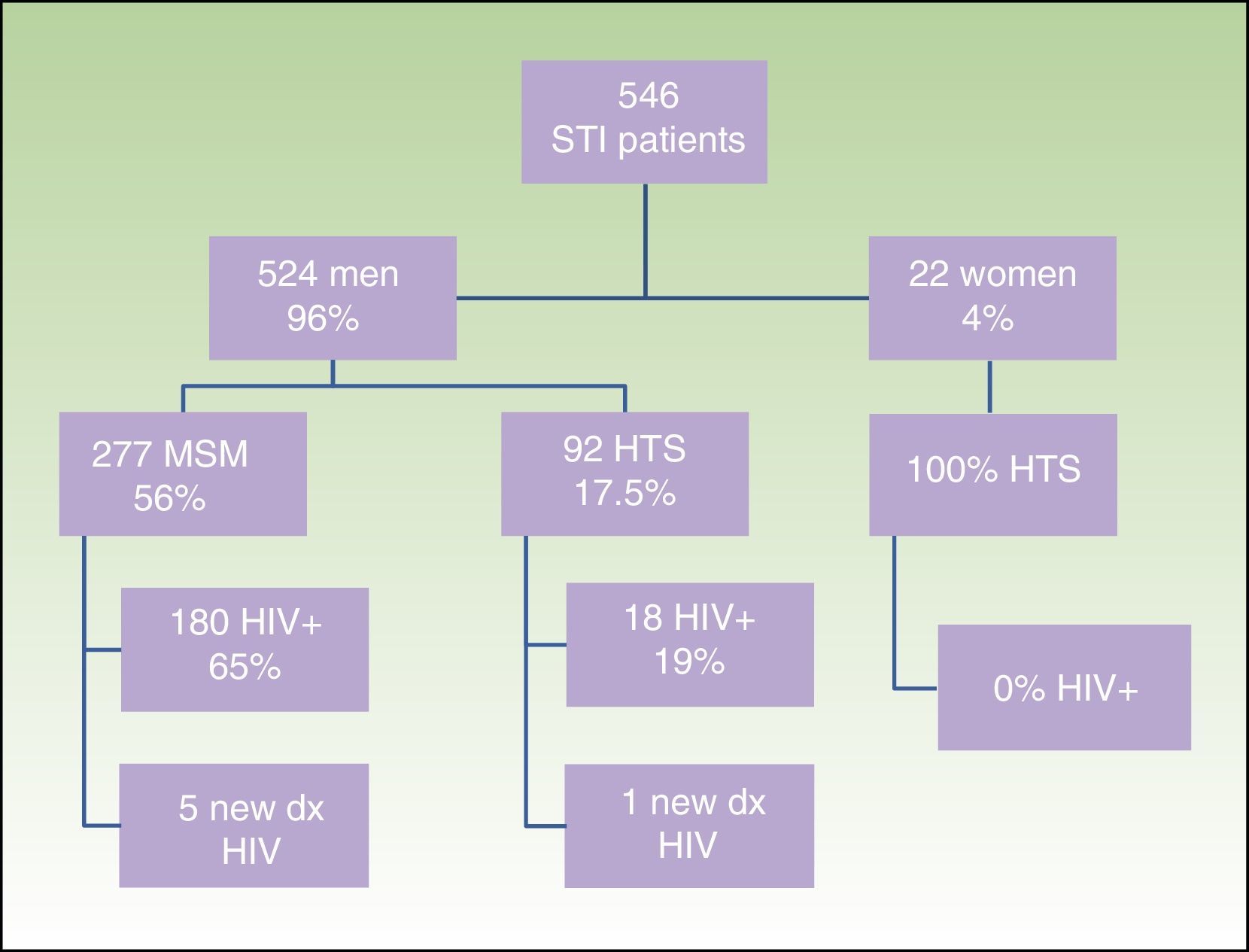
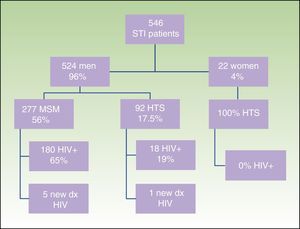
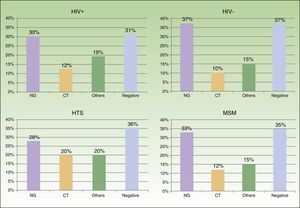
ResultsWe obtained epidemiological, behavioral, clinical, and microbiological data for 546 (65%) of the 835 patients seen at our STI unit between January 2010 and December 2013. In total, 144 of the patients were seen in 2010, 129 in 2011, 152 in 2012, and 121 in 2013; 96% (n=524) were men and 4% (n=22) were women. The mean age was 34 years. The patients’ ages ranged from 14 to 86 years, and a majority of patient (68.9%) were aged 20 to 39 years. Of the 546 patients seen, 198 (36%) were known to be HIV-positive and over 90% of these were already in the HIV registry held by the HCB's infectious diseases department. Over half of the patients (56%) were men who reported having sex with men (MSM), and 65% of these were HIV-positive; 17.5% reported being heterosexual, and 19% were positive for HIV infection. There were no data for the remaining 23% (Fig. 1).
Over 50% of patients reported having had 10 or more sexual partners in the previous year, and 32% reported taking drugs before sexual activity.
The main reasons for consultation were urethritis (n=148), genital and/or anal/perianal ulcers (n=82), balanoposthitis (n=39), genital/anal warts (n=13), oral ulcers (n=11), vulvovaginitis (n=4), enlarged inguinal lymph nodes (n=3), sexual relations with a partner diagnosed with an STI (n=41), and risky sexual behavior (n=19).
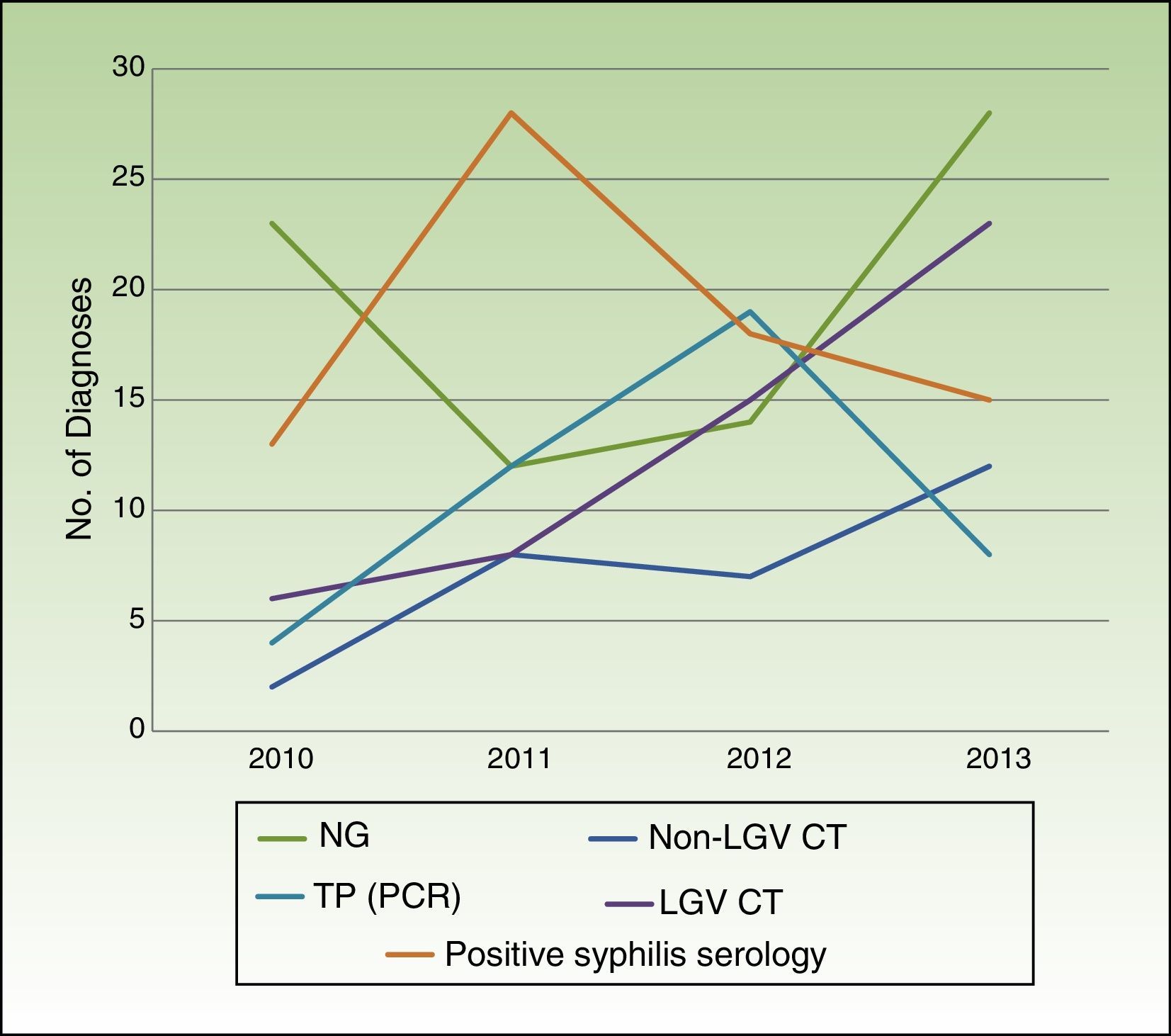
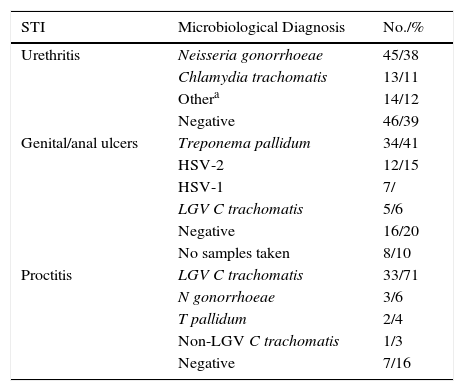
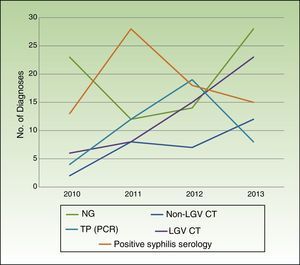
Table 2 summarizes the microbiological results according to clinical presentation. Changes in the number of cases for the main microbiological diagnoses over the study period are shown in Fig. 2.
Microbiological Results According to Clinical Presentation.
| STI | Microbiological Diagnosis | No./% |
|---|---|---|
| Urethritis | Neisseria gonorrhoeae | 45/38 |
| Chlamydia trachomatis | 13/11 | |
| Othera | 14/12 | |
| Negative | 46/39 | |
| Genital/anal ulcers | Treponema pallidum | 34/41 |
| HSV-2 | 12/15 | |
| HSV-1 | 7/ | |
| LGV C trachomatis | 5/6 | |
| Negative | 16/20 | |
| No samples taken | 8/10 | |
| Proctitis | LGV C trachomatis | 33/71 |
| N gonorrhoeae | 3/6 | |
| T pallidum | 2/4 | |
| Non-LGV C trachomatis | 1/3 | |
| Negative | 7/16 |
Abbreviations: HSV, herpes simplex virus; LGV, lymphogranuloma venereum; STI, sexually transmitted infection.
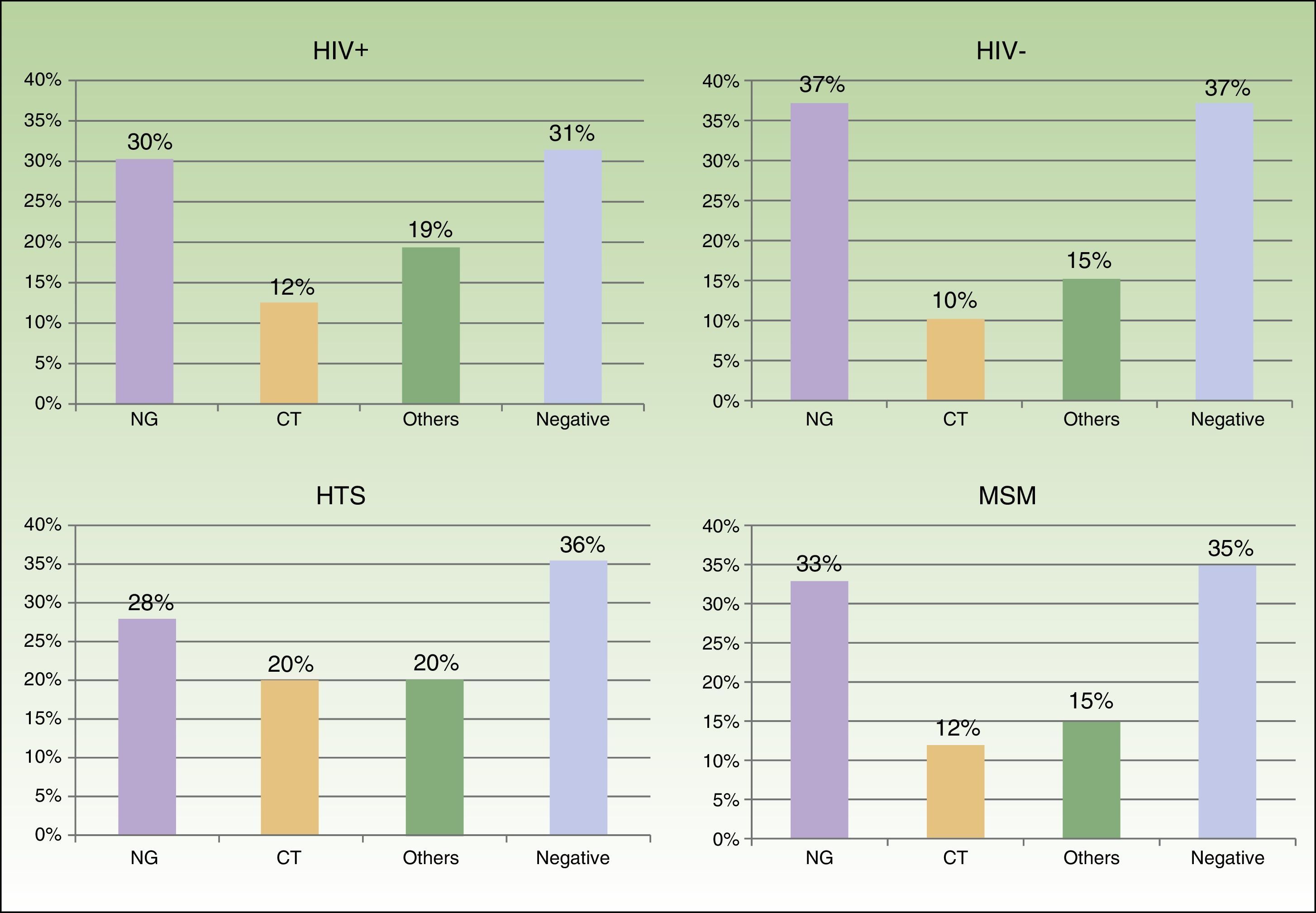
We saw 148 individuals with suspected urethritis, and samples were taken from 119 of these. The other 29 patients either had no clinical manifestations indicative of urethritis or had already received specific treatment. N gonorrhoeae was detected in 38% of patients, C trachomatis in 11% Haemophilus species in 8%, and Ureaplasma species in 3%; the tests in the remaining 39% of cases were negative. Gonococcal infection was the main cause of urethritis in all the groups studied; it was identified in 33% of MSM with urethritis, in 28% of heterosexuals, in 30% of HIV-positive patients, and in 37.5% of HIV-negative patients. Urethritis due to C trachomatis was more common in heterosexual men (20% of all cases of urethritis) than in MSM (12%), and similar in HIV-positive and HIV-negative patients (12% vs 10%) (Fig. 3).
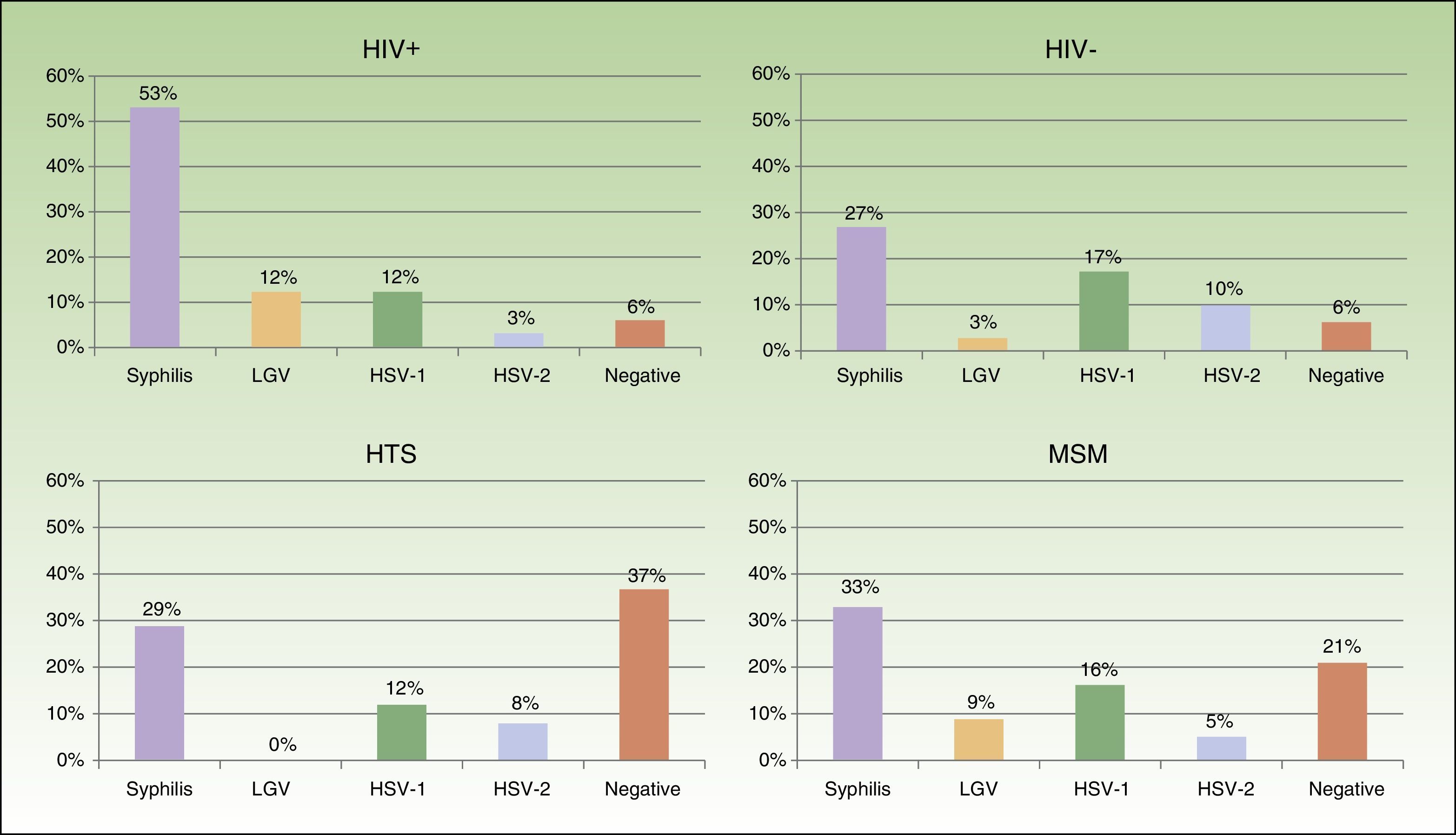
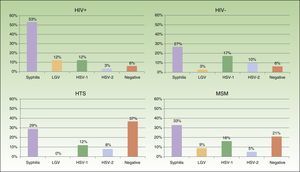
Eighty-two patients had genital and/or anal/perianal ulcers. T pallidum was detected in 41% of cases, HSV-2 in 15%, HSV-1 in 8%, and LGV C trachomatis in 6%. The culture was negative in 20% of cases. Clinical findings were sufficient to reach a diagnosis in 10% of patients. Genital and anal ulcers were mostly diagnosed in men (96%) (Fig. 4), with only 3 cases detected in women. Syphilis sores were more common in MSM (33%) than in heterosexuals (29%), in whom ulcers of a noninfectious origin predominated (37%); these sores were also more common in HIV-positive patients (53%) than in HIV-negative patients (27%). Primary syphilis was detected in 43 patients and secondary or latent syphilis in 74; 72.2% of syphilis cases corresponded to MSM and 17.6% to heterosexuals; 54.6% were positive for HIV infection and 33.6% were negative. We detected 10 extragenital sores, 8 oral sores, 1 tonsil sore, and 1 nipple sore, all in HIV-positive MSM. Herpes ulcers were found in a similar proportion of MSM (21%) and heterosexuals (20.5%), and were more common in HIV-negative patients than in HIV-positive patients (27% vs 15%). HIV-positive patients, however, had a higher proportion of ulcers due to LGV (12% vs 3% for HIV-negative patients).
Proctitis tests (n=46) revealed LGV C trachomatis in 71% of cases, N gonorrhoeae in 6%, T pallidum in 4%, and non-LGV C trachomatis in 3%; the culture was negative in 16% of cases. Six patients (1.1%) were newly diagnosed with HIV infection. All cases of proctitis were recorded in men, 96% of whom were HIV-positive and 98% of whom were MSM; the most common causes were LGV (42%) and gonorrhea (14%).
Twenty-five patients (4.6%) had more than 1 STI; the most common infections were urethritis due to N gonorrhoeae, proctitis due to LGV, and syphilis (primary and secondary). Fourteen of these patients (56%) had HIV coinfection.
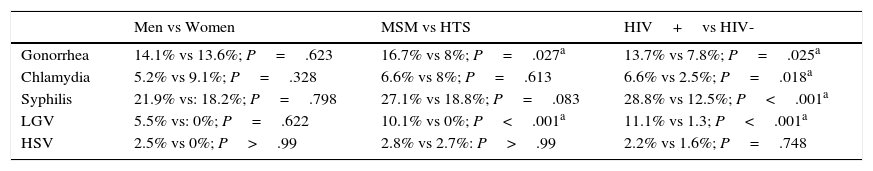
The statistical analysis showed a higher frequency of gonorrhea and LGV in MSM than in heterosexuals, but the rest of the STIs were evenly distributed in both groups. The STIs analyzed were also more common in HIV-positive than in HIV-negative patients, with the exception of HSV, which was similar in frequency in both groups (Table 3).
List of Most Common Sexually Transmitted Infections According to Sex, Sexual Orientation, and HIV Status.
| Men vs Women | MSM vs HTS | HIV+vs HIV- | |
|---|---|---|---|
| Gonorrhea | 14.1% vs 13.6%; P=.623 | 16.7% vs 8%; P=.027a | 13.7% vs 7.8%; P=.025a |
| Chlamydia | 5.2% vs 9.1%; P=.328 | 6.6% vs 8%; P=.613 | 6.6% vs 2.5%; P=.018a |
| Syphilis | 21.9% vs: 18.2%; P=.798 | 27.1% vs 18.8%; P=.083 | 28.8% vs 12.5%; P<.001a |
| LGV | 5.5% vs: 0%; P=.622 | 10.1% vs 0%; P<.001a | 11.1% vs 1.3; P<.001a |
| HSV | 2.5% vs 0%; P>.99 | 2.8% vs 2.7%: P>.99 | 2.2% vs 1.6%; P=.748 |
Abbreviations: LGV, lymphogranuloma venereum; HSV, herpes simplex virus.
Between 2008 and 2013, the Barcelona Public Health Authority received notification of 286 cases of LGV, 2356 cases of syphilis, and 2552 cases of gonorrhea.5 Of these, the STI unit at HCB managed 35 cases of LGV (12%), 119 cases of syphilis (5%), and 95 cases of gonorrhea (3.7%), making our hospital the tertiary care center with the highest caseload of patients with notified STIs in the area of Barcelona. For the purpose of this study, however, we were only able to obtain information for 65% of all patients seen at our unit between 2010 and 2013, showing that underreporting is still a problem for the epidemiological control of STIs in our setting,6 and one of the main limitations of our study. The drop in patients registered for 2013 reflects the reduction in resources made available to the unit during this period.
Coinciding with reports by CEEISCAT,1 our 4-year analysis shows a progressive increase in STIs due to LGV C trachomatis, non-LGV C trachomatis, and N gonorrhoeae. Syphilis diagnoses increased over the 4-year period, although the increase was less pronounced in the last 2 years, contrasting with findings by the CEEISCAT showing a progressive increase in reported cases in Catalonia over the same period.
The catchment area of our hospital includes an area of Barcelona with shops and nightclubs for MSM. A large proportion of the patients seen at our STI unit come from the HCB's infectious diseases day clinic, and approximately half were infected with HIV through sexual exposure. The population therefore differs from that described in other studies of STIs,2,7,8 as we see a large proportion of MSM and HIV-positive patients. There were few immigrants in our series, and we therefore did not perform a comparative analysis.
Studies of at-risk heterosexual individuals and asymptomatic patients in the United Kingdom and the Netherlands have shown C trachomatis to be the main cause of urethritis, particularly in women and young heterosexual males, and have led to the implementation of programs to screen for this infection in these countries.9,10 While C trachomatis was also a common cause of urethritis in heterosexual patients in our series, it was surpassed in frequency by N gonorrhoeae, which was the most common cause of urethritis in all the groups analyzed.
Most of the genital and anal ulcers reported in our series were detected in men. The most common cause was syphilis, particularly in MSM and HIV-positive patients, followed by HSV, which was more common in the HIV-negative population. A substantial proportion of heterosexual patients had ulcers of noninfectious origin. Ten extragenital syphilis sores were identified, all in HIV-positive MSM. This number is relatively low in comparison to the total number of syphilis sores observed in the genital region, but it shows that this disease must be contemplated in patients with acute extragenital cutaneous or mucosal ulcers who engage in risky sexual behavior.
All cases of LGV reported were diagnosed in MSM, and over 90% of these were HIV-positive. This proportion is higher than that described in 2013 by CEEISCAT, which reported HIV positivity in 65% of patients with LGV1; it is similar, however, to the rate of 94% reported by an epidemiological study of an LGV outbreak in Barcelona.10
Syphilis and gonorrhea were both significantly more common in MSM in the statistical analysis. The STIs analyzed were also significantly more common in HIV-positive than in HIV-negative patients, with the exception of HSV, which was similar in frequency in both groups.
In the 4 years analyzed, we found no cases of T vaginalis or H ducreyi infection, despite specific testing. As with C trachomatis infection, we believe that the low prevalence of trichomoniasis detected—which contrasts with rates reported by other studies in our setting9,11—can be explained by the epidemiological characteristics of our patients, and perhaps the low sensitivity of the technique employed.
Our findings support previous reports that HIV-positive MSM are particularly susceptible to STIs.12,13 As indicated by the epidemiological surveys conducted, probable reasons are multiple sexual partners, high frequency of sexual relations with anonymous partners, certain sexual practices, relaxation of safe-sex precautions (because of the widespread perception that there is no risk of HIV infection from individuals with an undetectable viral load), and use of illicit substances.
The limitations of our analysis are those inherent to a retrospective, descriptive study. The sample was not selected randomly and is not therefore representative of the general population. In addition, data from personal surveys are prone to social desirability and recall bias. We also did not routinely perform microbiological studies to rule out infection by M hominis or Ureaplasma urealyticum and therefore the true prevalence of these infections will have been underestimated. The same applies to human papillomavirus infection, which is not a considered an urgent presenting complaint in our unit. Finally, it would be desirable to conduct a more exhaustive assessment of the contacts of the patients seen at our STI unit, but this would require additional human and financial resources.
Our data confirm the epidemiological characteristics of the HIV/STI epidemic in our setting and highlight the fact that HIV-positive MSM are particularly susceptible to other STIs.
We consider that our findings provide interesting insights into the origin of STIs in patients who engage in high-risk sexual behaviors and highlight the role played by dedicated, multidisciplinary units in the control of these infections.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Moreno-Ribera N, Fuertes-de Vega I, Blanco-Arévalo JL, Bosch-Mestres J, González-Cordón A, Estrach-Panella T, et al. Infecciones de transmisión sexual: experiencia de una consulta multidisciplinar en un hospital terciario (2010-2013). Actas Dermosifiliogr. 2016;107:235–241.