Scarring alopecia refers to a group of disorders of various etiologies that cause permanent hair loss. In this article, we focus on primary cicatricial alopecia, a group of diseases in which the hair follicle is the main target of the inflammatory process. These disorders are currently classified as lymphocytic, neutrophilic, or mixed according to the cells that make up the inflammatory infiltrate. The pathogenesis of the majority of these conditions is not fully understood and they may have similar clinical features, often making it necessary to perform 1 or more skin biopsies in order to reach a diagnosis. Management depends on early and accurate diagnosis and aggressive treatment in some cases in order to prevent follicular destruction and scarring.
Las alopecias cicatriciales constituyen un grupo de trastornos que dan lugar a una pérdida permanente de cabello como consecuencia de diversos procesos. En este artículo nos centraremos en las alopecias cicatriciales primarias (ACP), un grupo de enfermedades foliculocéntricas en las que el folículo piloso es la principal diana del proceso inflamatorio. Actualmente se clasifican según la celularidad del infiltrado inflamatorio en linfocíticas, neutrofílicas y mixtas. La patogenia de muchas de ellas sigue siendo desconocida. Algunas presentan similitudes clínicas que dificultan el diagnóstico, lo que hace en muchos casos necesaria la práctica de una o más biopsias cutáneas. En el manejo de estas entidades es necesario un diagnóstico preciso de forma precoz y un tratamiento agresivo en algunos casos, con objeto de evitar la destrucción folicular y el desarrollo de una alopecia cicatricial.
The term scarring alopecia (or cicatricial alopecia) refers to a group of disorders that cause permanent hair loss when hair follicles are obliterated by fibrosis or hyalinized collagen.
The destruction of the hair follicle may be secondary to an inflammatory process, such as kerion, trauma, or radiation therapy, or can be caused by a carcinoma or metastatic tumor. However, the term cicatricial alopecia is used preferentially to refer to the primary cicatricial alopecias, a group of folliculocentric disorders in which the hair follicle is the main target of an inflammatory process that spares the interfollicular dermis.1 In this article, we will focus mainly on this group of primary alopecias, which often represent a clinical and therapeutic challenge.
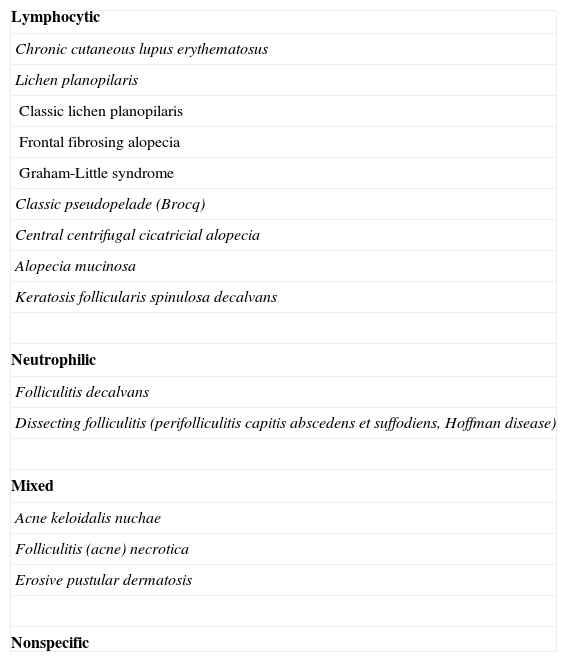
Primary Cicatricial AlopeciasPrimary cicatricial alopecias account for around 3% of all cases of alopecia seen in the dermatology department, although no data are available on the exact prevalence of this condition in the general population. A number of classification schemes have been proposed and the current system classifies these disorders into 3 groups (lymphocytic, neutrophilic, and mixed) according to the predominant cell type present in the inflammatory infiltrate (Table 1).2
Classification of Cicatricial Alopecias.
| Lymphocytic |
| Chronic cutaneous lupus erythematosus |
| Lichen planopilaris |
| Classic lichen planopilaris |
| Frontal fibrosing alopecia |
| Graham-Little syndrome |
| Classic pseudopelade (Brocq) |
| Central centrifugal cicatricial alopecia |
| Alopecia mucinosa |
| Keratosis follicularis spinulosa decalvans |
| Neutrophilic |
| Folliculitis decalvans |
| Dissecting folliculitis (perifolliculitis capitis abscedens et suffodiens, Hoffman disease) |
| Mixed |
| Acne keloidalis nuchae |
| Folliculitis (acne) necrotica |
| Erosive pustular dermatosis |
| Nonspecific |
While the pathogenesis of primary cicatricial alopecias remains poorly understood, recent studies indicate that the pathogenic process targets stem cells of the follicular bulge, the inner and outer sheaths, and the infundibulum.3 Harries and Paus4 have suggested that the critical factor may be that these stem cells can reach a point of no return beyond which the hair follicle is irreversibly damaged. Under normal conditions, hair follicle epithelial stem cells appear to enjoy a certain degree of autoimmune protection because they are located in an immunologically privileged niche. In some forms of primary cicatricial alopecia, such as lichen planopilaris and chronic cutaneous lupus erythematosus, this protection may collapse.4 Another hypothesis is that the folliculocentric inflammation that destroys the follicle is caused by a cytotoxic autoimmune mechanisms against the follicular autoantigens. Defects in sebaceous gland function and interference in the communication between hair follicle mesenchyme and epithelium have also been cited as possible mechanisms.5
Clinical FeaturesTypically, alopecic plaques are asymptomatic and extend slowly; however, in some cases progress is more rapid and accompanied by itching, burning, and pain. Hair loss is irreversible. The center of the plaques is characterized by the disappearance of the skin markings and the loss of follicular ostia while follicular hyperkeratosis and perifollicular erythema are seen at the hair-bearing margins. The plaques are poorly defined and the surface of the skin is smooth with no scaling. In some cases, inflammation and even suppuration are apparent, especially in the neutrophilic forms of the disease.
PathophysiologyA biopsy is recommended to confirm diagnosis and classify the type of disease. The specimen should be cut parallel to the hair shaft rather than slicing through the follicle and hematoxylin-eosin staining should be used. The site chosen for skin biopsy is of fundamental importance because it will determine the usefulness of the results of histopathology. The stage of development of the lesion analyzed is also crucial and it is preferable to biopsy a clinically active lesion in the early stages because the yield from scar lesions is low. If only 1 biopsy specimen is taken, most experts recommend histologic examination of transverse sections as this method allows visualization of multiple hair follicles at different stages of growth.6–8 Other authors consider 2 biopsies desirable to facilitate simultaneous analysis of transverse and vertical sections. Elastin stains (Verhoeff-van Gieson) can be useful to identify end-stage lesions and confirm the presence of scarring.9
In a study of 136 biopsy specimens of scarring alopecia of the scalp, the most frequent diagnosis was lichen planus (26%), followed by chronic cutaneous lupus erythematosus (21%), folliculitis decalvans (20%), and Brocq pseudopelade (10%).10
Lymphocytic Primary Cicatricial AlopeciasThe main primary cicatricial alopecias of the lymphocytic type are chronic cutaneous lupus erythematosus and lichen planopilaris. The group also includes Brocq pseudopelade, a condition that may in fact represent the end stage of many of these alopecias. To confirm diagnosis, it may be useful to analyze a biopsy specimen using direct immunofluorescence, a technique that will reveal a continuous band of immunoglobulin (Ig) G in about 50% of cases of chronic cutaneous lupus erythematosus.
Chronic Cutaneous Lupus Erythematosus or Discoid Lupus ErythematosusBetween 30% and 50% of patients with discoid lupus erythematosus have scalp involvement. In fact the scalp may be the first and only site affected in one-third of patients with lupus. The condition is 2 to 5 times more common in women.11
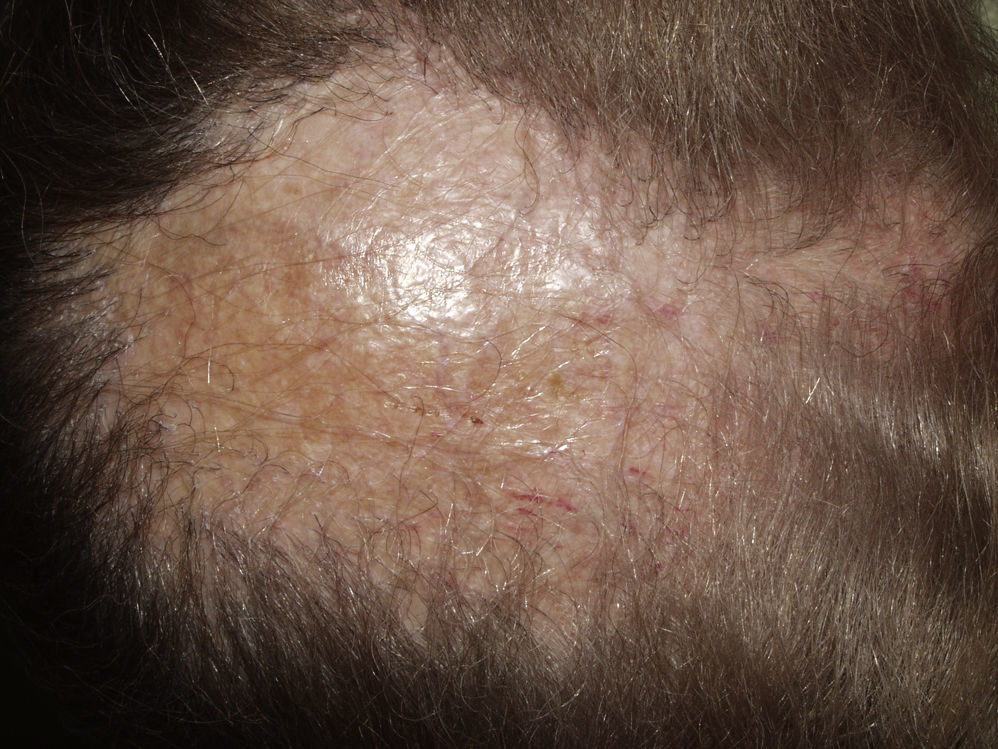
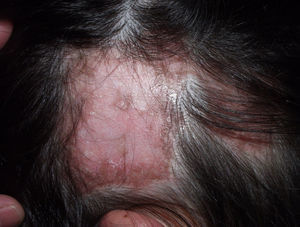
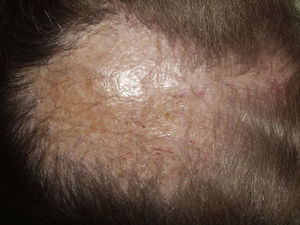
Typically, the first manifestation is an erythematous plaque. Disease spreads centrifugally to form a characteristic discoid or coin-shaped plaque covered with scales that are attached to the skin by horny plugs. Symptoms include itching, burning, and pain. The disease progresses with scarring and atrophy in the center of the plaque, (Fig. 1) which is characterized by hair loss and obliteration of the follicular ostia. Peripheral erythema, telangiectasia, and hyperkeratosis are seen at the hair-bearing margins. Specimens obtained from active lesions will show vacuolization of the basal follicular layer, dyskeratotic keratinocytes, and a dense perivascular and periadnexal lymphocytic infiltrate. Findings in the interfollicular epithelium include orthokeratotic hyperkeratosis with horny plugs, epidermal atrophy, basal vacuolization with cytoid bodies, and thickening of the basement membrane. Dermal mucin is also frequently observed. Direct immunofluorescence assay detects deposition of IgG, IgM, and complement in over 70% of cases.
The first step in the treatment of discoid lupus erythematosus is to rule out the presence of systemic lupus erythematosus on the basis of a complete medical history and the results of antinuclear antibody and kidney function tests. Discoid lupus erythematosus is treated with similar therapies whether it affects the scalp or the skin.11–13 The standard treatment is twice-daily application of topical high-potency corticosteroids, such as betamethasone dipropionate 0.05% or clobetasol propionate 0.05% in cream, gel, lotion, or foam (Table 2) and deep dermal injection every 4 to 6 weeks of 4 to 10mg/mL of triamcinolone acetonide. The solution is prepared by dissolving 0.1 to 0.2mL from a 40-mg vial of triamcinolone acetonide in 0.8 to 0.9mLof saline solution. The resulting dilution is then injected into the plaques (0.1 mL per cm2, maximum 2 mL) with an insulin syringe and needle. Treatment with potent topical corticosteroids can leave permanent areas of hypopigmentation and telangiectasia and the injections may cause atrophy. If there is no response within 8 to 12 weeks, oral antimalarial agents may be added. Antimalarials are the first-line option in the case of rapidly progressive alopecia. Treatment is usually started with hydroxychloroquine because of its lower ocular toxicity14 and patients should have a baseline eye examination. Smokers should be advised to stop smoking before starting treatment because it has been observed that tobacco diminishes response to treatment in a dose-dependent fashion.15 The initial dose of hydroxychloroquine is 200 to 400mg/d (4-6mg/kg/d in children), and improvement is usually observed within 4 to 8 weeks. A certain degree of repopulation of the hair can even be achieved if treatment is started promptly. If the outbreak is very severe, oral prednisone 1mg/kg can be added until disease is controlled, gradually tapering the dose over 8 weeks. In selected cases a combination of antimalarial drugs may be indicated because of their synergistic effect.16 Treatment should be maintained over a prolonged period until a few weeks after the inflammation has resolved, when the result of gradual withdrawal can be tested. In cases of resistant disease, treatment with oral retinoids may be useful. Even though acitretin and hydroxychloroquine have been shown to be equally effective,14 it is preferable to start with isotretinoin at a dosage of 1mg/kg/d17 because it causes less telogen effluvium. Moreover, isotretinoin has a shorter half-life, which reduces the risk of teratogenicity in women of childbearing age. In some cases, low maintenance doses are needed to control the disease over a prolonged period (10-40mg/d). Marked improvement has also been reported with thalidomide at doses between 50 and 300mg/d, although low maintenance doses of 25 to 50mg/d are usually required because relapse occurs early and frequently.18 The main adverse effects of this drug are teratogenicity and peripheral neuropathy.
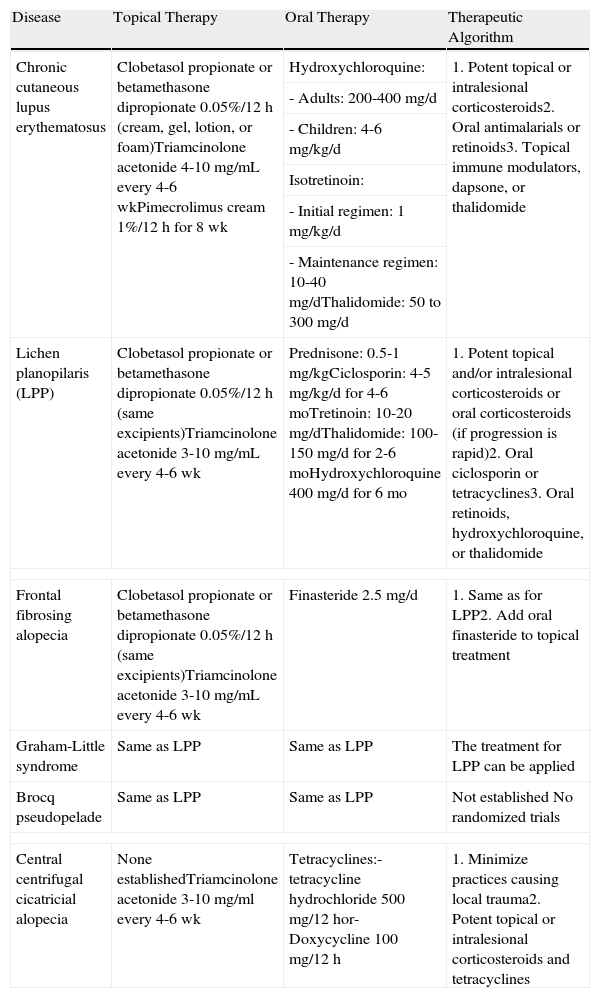
Treatment of Lymphocytic Primary Cicatricial Alopecias.
| Disease | Topical Therapy | Oral Therapy | Therapeutic Algorithm |
| Chronic cutaneous lupus erythematosus | Clobetasol propionate or betamethasone dipropionate 0.05%/12 h (cream, gel, lotion, or foam)Triamcinolone acetonide 4-10mg/mL every 4-6 wkPimecrolimus cream 1%/12 h for 8 wk | Hydroxychloroquine: | 1. Potent topical or intralesional corticosteroids2. Oral antimalarials or retinoids3. Topical immune modulators, dapsone, or thalidomide |
| - Adults: 200-400 mg/d | |||
| - Children: 4-6 mg/kg/d | |||
| Isotretinoin: | |||
| - Initial regimen: 1 mg/kg/d | |||
| - Maintenance regimen: 10-40 mg/dThalidomide: 50 to 300 mg/d | |||
| Lichen planopilaris (LPP) | Clobetasol propionate or betamethasone dipropionate 0.05%/12 h (same excipients)Triamcinolone acetonide 3-10 mg/mL every 4-6 wk | Prednisone: 0.5-1 mg/kgCiclosporin: 4-5 mg/kg/d for 4-6 moTretinoin: 10-20 mg/dThalidomide: 100-150 mg/d for 2-6 moHydroxychloroquine 400 mg/d for 6 mo | 1. Potent topical and/or intralesional corticosteroids or oral corticosteroids (if progression is rapid)2. Oral ciclosporin or tetracyclines3. Oral retinoids, hydroxychloroquine, or thalidomide |
| Frontal fibrosing alopecia | Clobetasol propionate or betamethasone dipropionate 0.05%/12 h (same excipients)Triamcinolone acetonide 3-10 mg/mL every 4-6 wk | Finasteride 2.5 mg/d | 1. Same as for LPP2. Add oral finasteride to topical treatment |
| Graham-Little syndrome | Same as LPP | Same as LPP | The treatment for LPP can be applied |
| Brocq pseudopelade | Same as LPP | Same as LPP | Not established No randomized trials |
| Central centrifugal cicatricial alopecia | None establishedTriamcinolone acetonide 3-10 mg/ml every 4-6 wk | Tetracyclines:- tetracycline hydrochloride 500 mg/12 hor- Doxycycline 100 mg/12 h | 1. Minimize practices causing local trauma2. Potent topical or intralesional corticosteroids and tetracyclines |
Abbreviation: LPP, lichen planopilaris.
Some studies have also shown improvement in the lesions of discoid lupus erythematosus with topical immune modulators, such as pimecrolimus 1% cream applied twice daily for 8 weeks19,20 and tacrolimus 0.1%. In a comparative study of tacrolimus 0.1% and clobetasol propionate 0.05%, no significant differences in effectiveness between the 2 treatments were found.21 However, the usefulness of these results is debatable owing to the small number of patients in the study.
Patients must also be advised to avoid exposure to sunlight during peak hours and to use photoprotective measures in the form of a wide-brimmed hat, clothes that cover the neck and shoulders, and regular application of sunscreen with a high solar protection factor.
Other drugs that have been used to treat discoid lupus erythematosus are dapsone, mycophenolate mofetil, azathioprine, gold, clofazimine, and tazarotene.11,22,23 Some authors have expressed concern about the use of biologic agents in lupus erythematosus because it has been observed that anti-TNF drugs such as etanercept may induce lupus.24 Biologic agents also favor the appearance of circulating antibodies and predispose patients to infection by mycobacteria.
Lichen PlanopilarisLichen planopilaris is the name given to lichen planus when it affects the hair follicles. It is the most common primary cicatricial alopecia. In addition to the classic form, there are 3 variants of lichen planopilaris: frontal fibrosing alopecia, central centrifugal cicatricial alopecia, and Graham-Little syndrome. Lichen planopilaris usually begins on the vertex or the parietal area and is more common in middle-aged women. Follicular lichen planus sometimes occurs in association with typical lichen planus lesions affecting the skin, nails, or mucous membranes.25
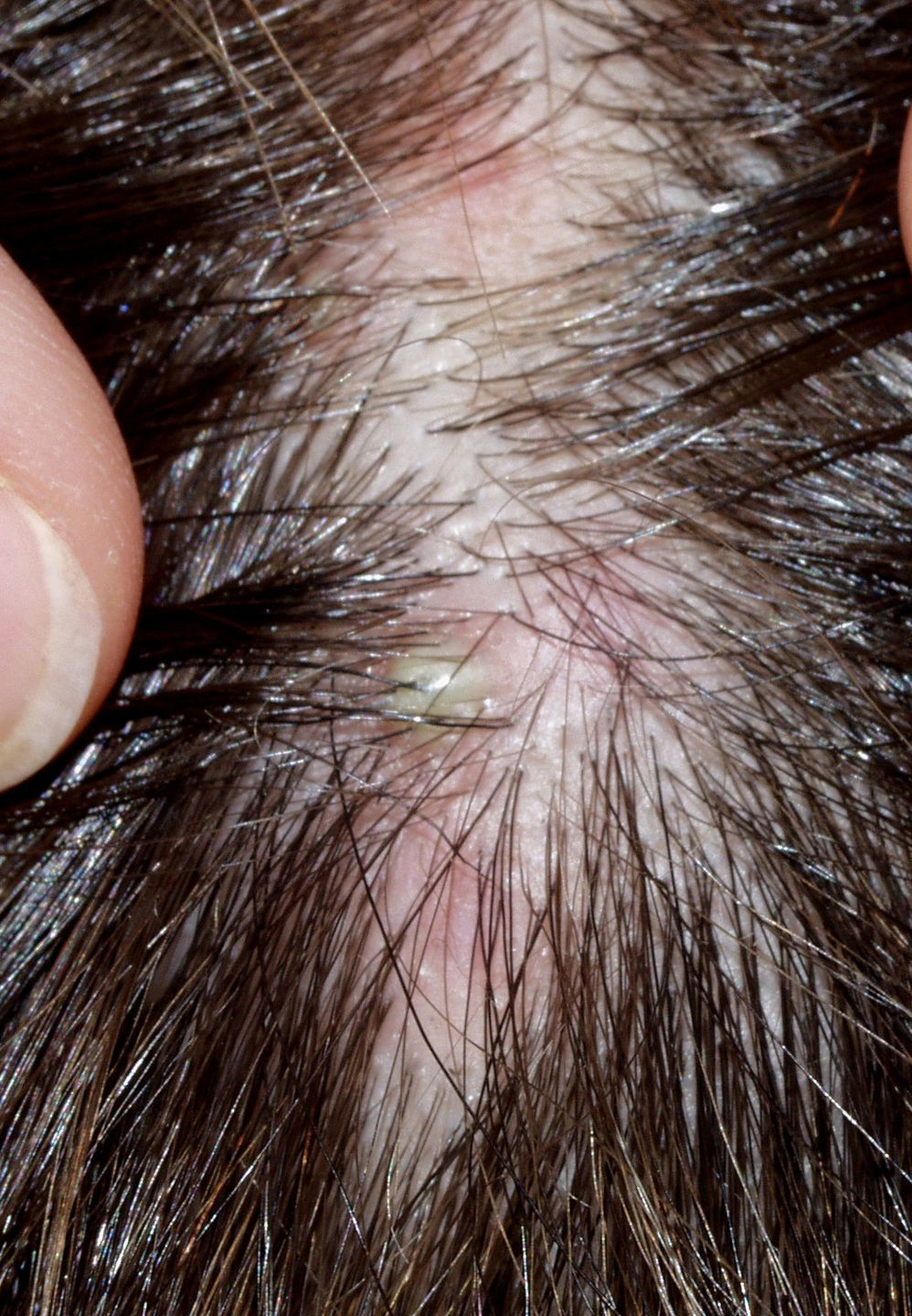
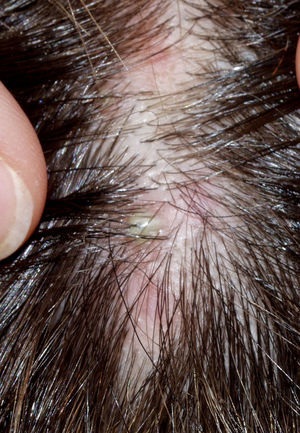
The condition is characterized by perifollicular keratotic papules and spinous follicular hyperkeratosis. The plaques progress to scarring alopecia (Fig. 2), which is generally multifocal caused by several simultaneous lesions. Patients usually report itching, burning, or pain on the scalp. Follicular openings with single hairs are seen in the center of some plaques while follicles containing groups of 2 or 3 hairs (tufted folliculitis) are observed in others. The skin on the plaques becomes parchment-like. The edges of the plaques are poorly defined and the skin in the center is smooth with no scaling. Hair loss is irreversible.
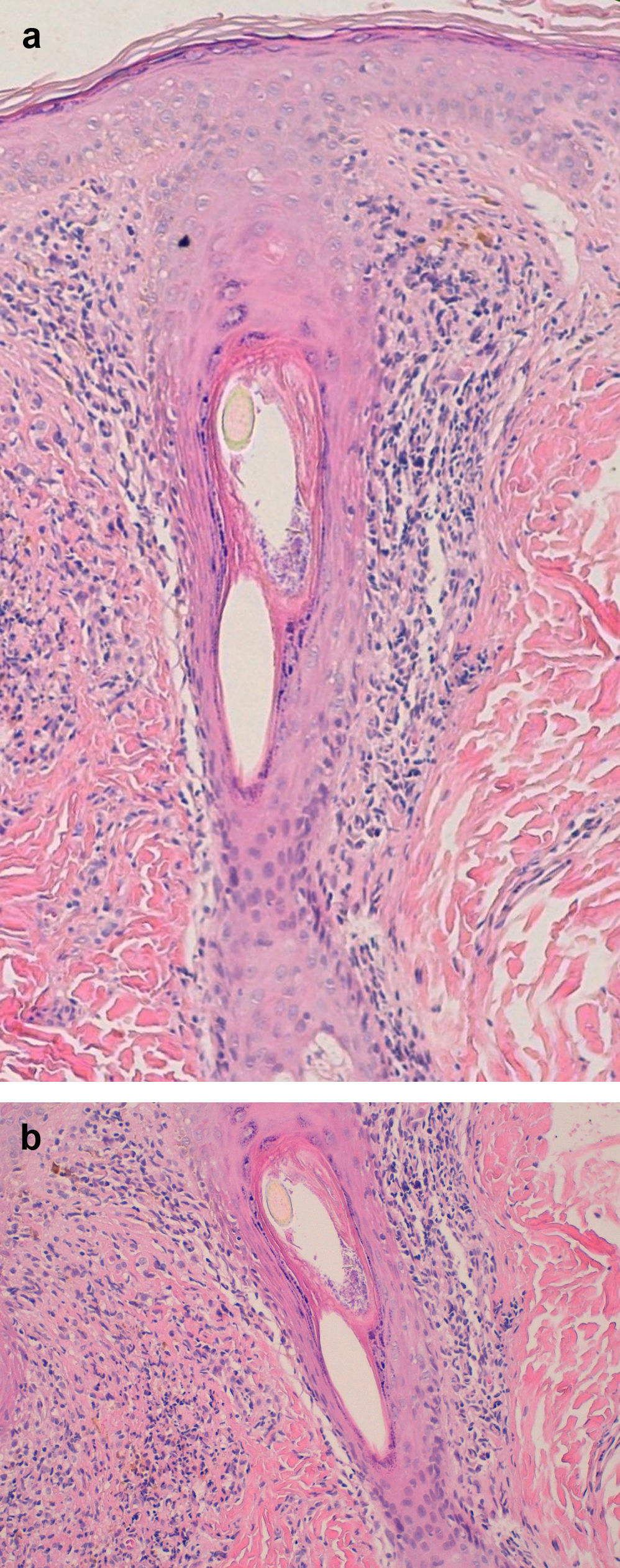
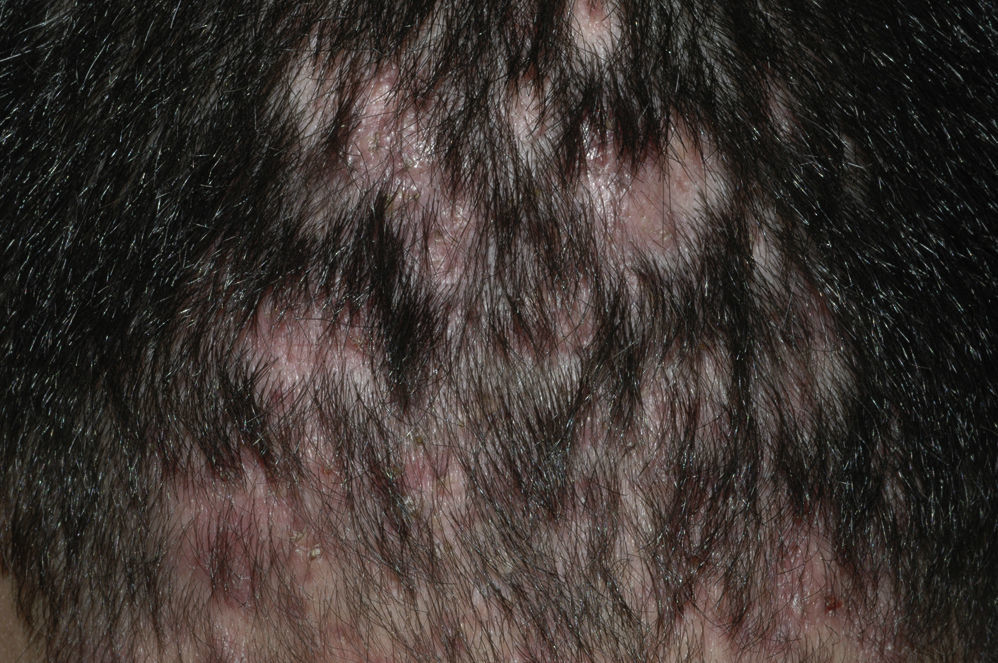
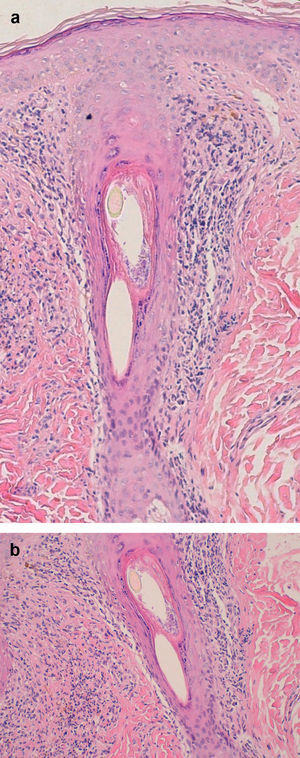
Examination of a biopsy specimen reveals a predominantly perifollicular lymphocytic infiltrate (Fig. 3) in the reticular dermis. In the mid-dermis a mucinous perifollicular fibroplasia is observed but no noticeable interfollicular mucin. Superficial perifollicular wedge-shaped scarring is also observed. Also characteristic is the absence of sebaceous glands and the arrector pili muscle.26
Before starting any treatment, the first step is to rule out the possibility that the patient's condition is induced by a drug—in particular gold,27 atabrine, or quinacrine—or by vaccination for hepatitis B.28 Hepatitis C virus infection must also be ruled out.
Initial treatment is usually either potent topical corticosteroids29,30 (Table 2) or injections of triamcinolone acetonide (3-10mg/mL, maximum 2mL) every 4 to 6 weeks, or a combination of both.25 Good results are obtained in 40% of cases of mild or moderate disease.13 In a study of 30 patients, 2 out of 3 patients achieved remission of symptoms with a 12-week course of topical corticosteroids using a gradually tapered dose regimen.30 The authors of another study reported partial and temporary improvement in 70% of patients.31 Systemic therapy should be reserved for treating rapidly progressive disease that fails to respond to topical corticosteroid treatment. Prednisone can be used for short periods of time at a dose of 0.5 to 1mg/kg to control the disease, with gradual tapering over 2 to 4 months.30,31 Oral ciclosporin has been used as an alternative in cases of refractory lichen planopilaris. In a series of 13 cases, an average dose of 4 to 5mg/kg/d for 4 to 6 months resulted in complete remission in 53% of patients and partial response in 23%.32 Despite the good results obtained with oral corticosteroids and ciclosporin, relapse upon withdrawal of treatment tends to occur in 60% to 80% of cases.31,33 Retinoids can also be used; marked improvement in 2 patients with lichen planopilaris was reported following treatment with low doses of oral tretinoin over several months.34
There is anecdotal evidence of the use of other treatments. In a retrospective study, disease stabilized in 6 of 11 patients treated with tetracyclines,35 a result that has led some authors to consider oral tetracyclines to be the treatment of choice because of their superior safety profile.
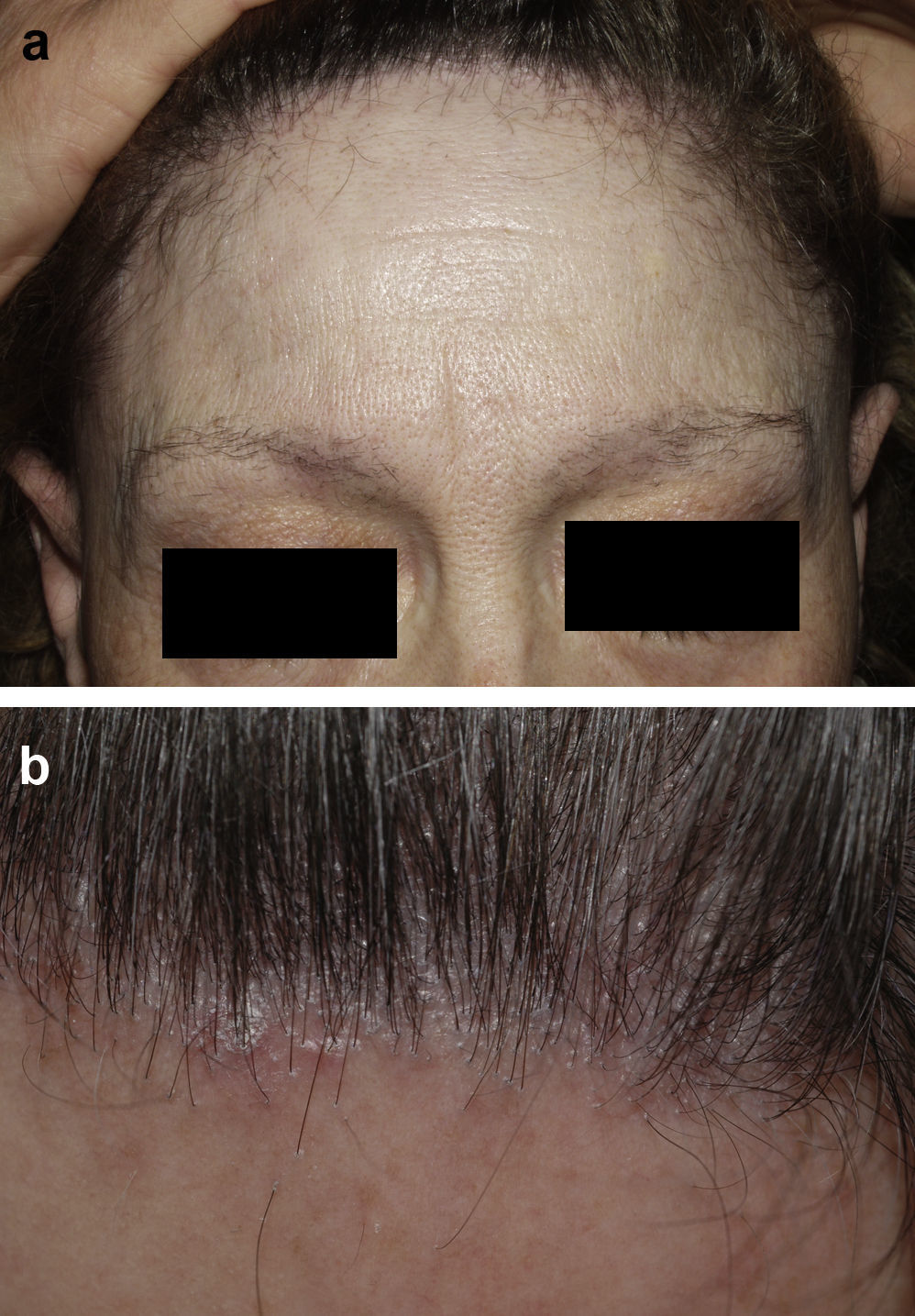
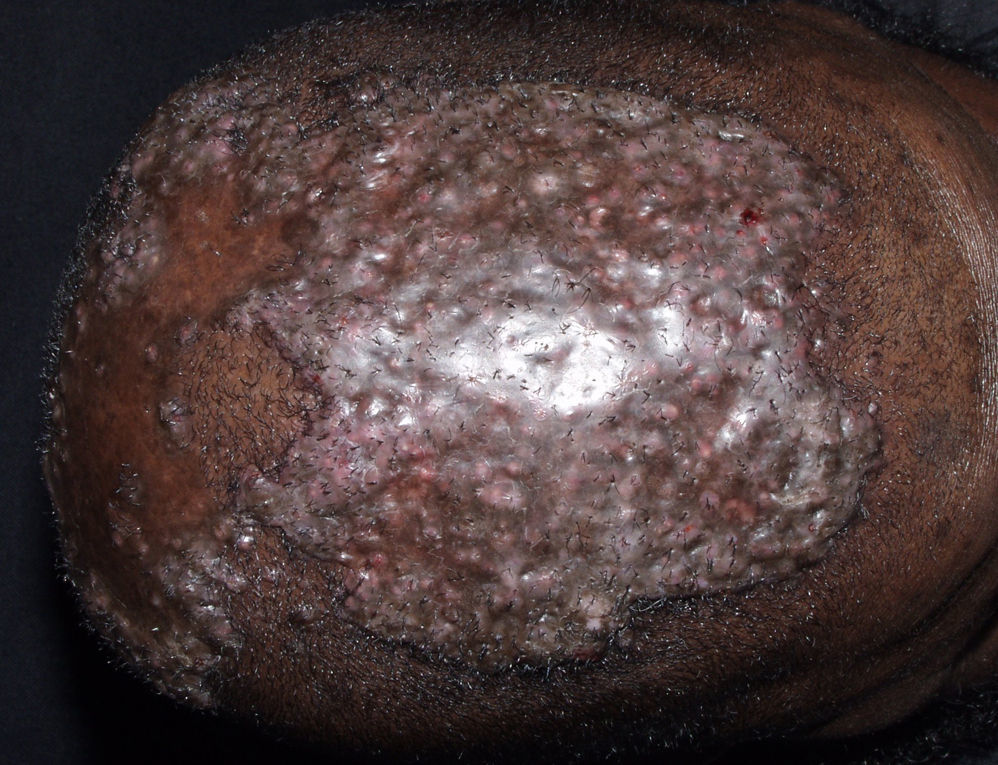
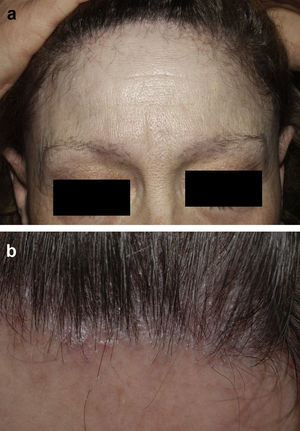
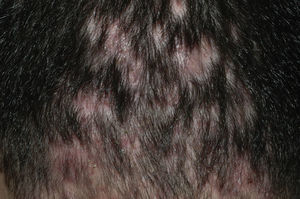
Frontal Fibrosing AlopeciaFrontal fibrosing alopecia refers to progressive recession of the frontal and temporal hairline accompanied, in some cases, by thinning of the eyebrows and sideburns. This condition primarily affects postmenopausal women (Fig. 4).36 Because of its clinical features (follicular hyperkeratosis and perifollicular erythema) and histologic characteristics (perifollicular lymphocytic infiltrate and lamellar fibrosis of the upper portion of the follicle), most experts believe that this condition is a variant of lichen planopilaris.37 No effective treatment has been reported, although during the hyperkeratotic phase disease appears to improve with medium- to high-potency topical corticosteroids. The therapeutic options recommended for the treatment of lichen planopilaris38,39 can be tried with the addition of oral finasteride to the topical corticosteroid therapy in women with typical female pattern hair loss. Monotherapy with oral finisteride is another option. It should be noted that finasteride is contraindicated in women of childbearing age.
Graham-Little SyndromeThis syndrome is characterized by the triad of patchy cicatricial alopecia of the scalp (similar to that caused by lichen planus), nonscarring loss of axillary and pubic hair, and clusters of spinous follicular papules on the trunk and limbs40,41 similar to those seen in lichen spinulosus. The treatment options are the same as for lichen planopilaris; treatment is challenging and response variable. Some authors have reported cases in which ciclosporin and thalidomide have been effective.41,42
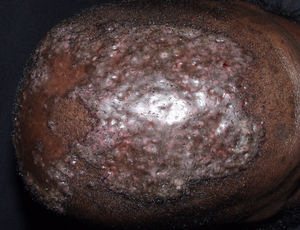
Central Centrifugal Cicatricial AlopeciaThere are probably 2 variants of central centrifugal cicatricial alopecia. The most typical form, found primarily in black women,43,44 is attributed to harsh hair-care practices, including the use of traumatic combing, heat, traction, and chemical products. The other form, more often found in white patients, can be considered a variant of lichen planus (Fig. 5). The fact that both forms share several clinical and histologic features with Brocq pseudopelade and folliculitis decalvans45 has led some authors to suggest that central centrifugal cicatricial alopecia may not be a separate entity but rather a clinical pattern shared by different scarring alopecias.
During the active phase, histology reveals lamellar fibroplasia and a perifollicular lymphocytic infiltrate affecting the upper portion of the hair follicle. In more advanced stages, a granulomatous perifollicular inflammation with foreign body giant cells is observed.46
The recommended treatment is avoidance of traumatic hair-care practices, if applicable, and, during the inflammatory phase, application of potent topical corticosteroids alone or in combination with oral tetracyclines (tetracycline hydrochloride 500mg/12h or doxycycline 100mg/12h).47 Treatment results in rapid improvement that can be maintained for several months, although most patients experience several relapses over a few years before the disease resolves completely, leaving oval scarred plaques, which can measure up to 10cm in length.
Brocq PseudopeladeBrocq pseudopelade is a chronic asymptomatic primary cicatricial alopecia most often involving the vertex. Brocq originally described 3 variants: scattered small plaques, scattered large plaques, and mixed type.48 Lesions take the form of pearly-colored or hypopigmented slight depressions sometimes described as “footprints in the snow.” There is much debate about whether Brocq pseudopelade is actually a separate entity or the final, postinflammatory stage of other forms of scarring alopecia. No well-established treatment protocol exists. Potent corticosteroids, hydroxychloroquine, and thalidomide have all been used. In all cases, the level of evidence is low owing to the lack of consensus on the nature of the disease and the absence of randomized trials or large series evaluating the efficacy of these treatments.
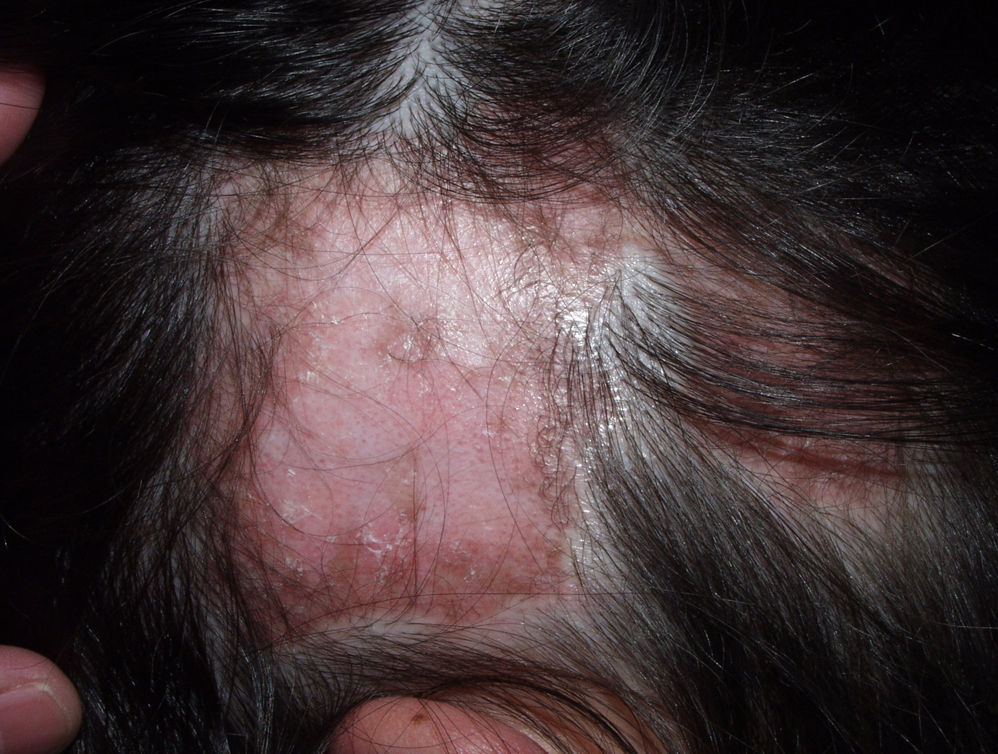
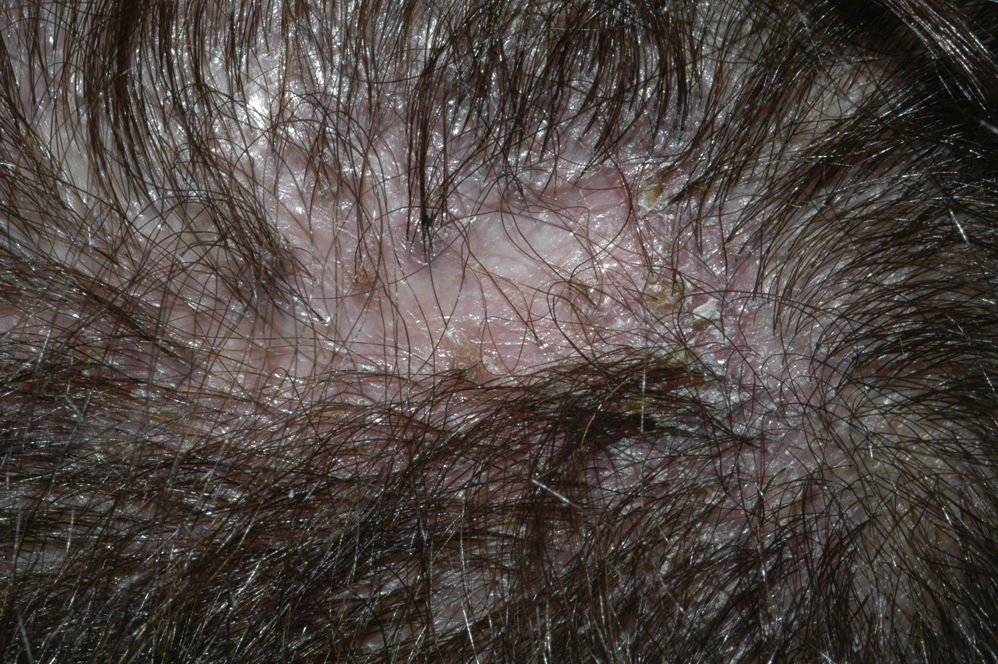
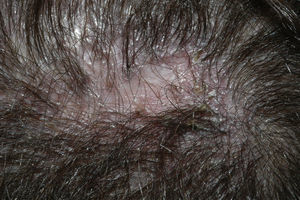
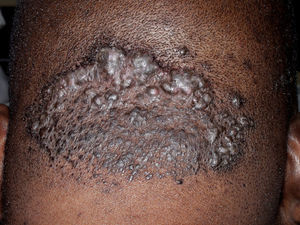
Neutrophilic Cicatricial AlopeciasFolliculitis DecalvansFolliculitis decalvans is a rare disorder characterized clinically by follicular pustules which are usually very painful (Fig. 6). Crops of new papulopustules and nodules may appear around the initial lesions, leaving in their wake irregularly-shaped, atrophic, flesh-colored patches of scarring alopecia (Fig. 7).49 The course of the disease is generally slow and chronic. This condition is more common in young and middle-aged patients and affects all races and both sexes equally. In most cases, Staphylococcus aureus is isolated in cultures,50 and most patients are nasal carriers of staphylococci.
In the initial stages, histologic examination reveals acneiform infundibular dilation and an intrafollicular and perifollicular neutrophilic infiltrate in the upper and middle portions of the hair follicle.51,52 In more advanced lesions, the infiltrate is mixed (neutrophils, lymphocytes, and plasma cells) and extends deeper into the adventitial dermis. However, the prominent abscesses and sinus tracts typical of dissecting folliculitis are not seen.
TreatmentThe first step in treatment should be to culture the pustules and obtain a sensitivity report. In patients with a history of recurrent infections; immunodeficiency must be ruled out. Folliculitis decalvans responds well to antistaphylococcal antibiotics (Table 3), cloxacillin, fusidic acid, clindamycin alone or in combination with rifampicin or amoxicillin clavulanate,53,54 and to broad-spectrum antibiotics and antineutrophil agents. However, the condition usually recurs when treatment is discontinued. In recent years, remissions of 2 years or more have been achieved with the introduction of combination therapies based on rifampicin53 because of their excellent bactericidal action and improved intracellular penetration.55,56 Rifampicin has been reported to eliminate nasal carriage of S aureus.57 Patients using this drug must be warned about the orange coloration they may observe in their urine and the possibility of developing a hypersensitivity syndrome with eosinsophilia.56,58,59 However, the use of rifampicin monotherapy should be avoided because this regimen facilitates the development of resistance.57 One of the most commonly used combinations is rifampicin (300mg/12h) + clindamycin (300mg/12h) for 10 weeks. Clindamycin can cause diarrhea and even pseudomembranous colitis caused by Clostridium difficile, a bacteria that is becoming increasingly aggressive.60 Rifampicin can also be used in combination with ciprofloxacin or clarithromycin. Oral fusidic acid appears to be equally effective alone or in combination with fusidic acid ointment (500mg/d for 3 weeks).61 Prolonged treatment with fusidic acid can cause gastrointestinal disorders.62 Liver function should be monitored, and this therapy should be avoided in patients with liver disease. Very good results have been obtained with broad-spectrum antibiotics, such as tetracyclines and trimethoprim-sulfamethoxazole. If these are used, a topical antibiotic, such as fusidic acid or mupirocin, should be added to eradicate nasal S aureus.63 Isotretinoin does not appear to be very effective, although 1 patient responded to a combination regimen of isotretinoin, prednisolone, and clindamycin.63 Some cases have responded to treatment with oral dapsone at doses of 75 to 100mg/d,64 but this treatment must be maintained over a prolonged period at a low dose to prevent relapse. Laser hair removal and radiotherapy at anti-inflammatory doses have also been tested with good results.65,66 Several treatment cycles may be necessary to achieve prolonged remission.
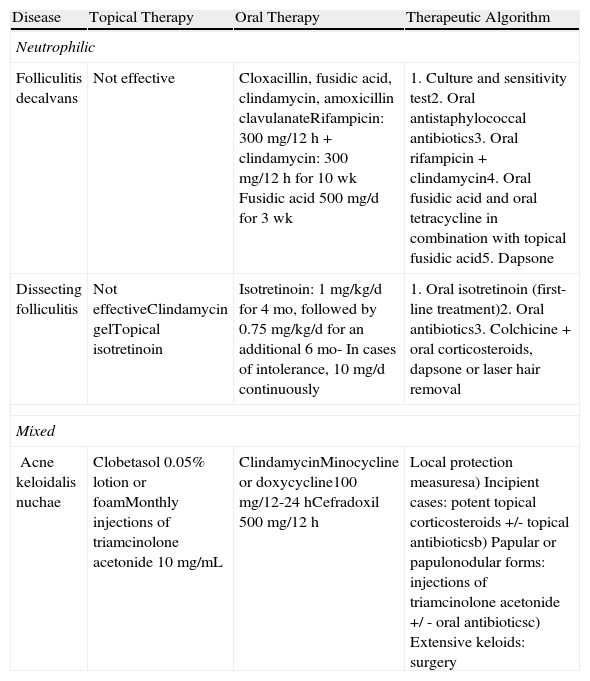
Treatment of Neutrophilic and Mixed Primary Cicatricial Alopecias.
| Disease | Topical Therapy | Oral Therapy | Therapeutic Algorithm |
| Neutrophilic | |||
| Folliculitis decalvans | Not effective | Cloxacillin, fusidic acid, clindamycin, amoxicillin clavulanateRifampicin: 300 mg/12 h + clindamycin: 300 mg/12 h for 10 wk Fusidic acid 500 mg/d for 3 wk | 1. Culture and sensitivity test2. Oral antistaphylococcal antibiotics3. Oral rifampicin + clindamycin4. Oral fusidic acid and oral tetracycline in combination with topical fusidic acid5. Dapsone |
| Dissecting folliculitis | Not effectiveClindamycin gelTopical isotretinoin | Isotretinoin: 1 mg/kg/d for 4 mo, followed by 0.75 mg/kg/d for an additional 6 mo- In cases of intolerance, 10 mg/d continuously | 1. Oral isotretinoin (first-line treatment)2. Oral antibiotics3. Colchicine + oral corticosteroids, dapsone or laser hair removal |
| Mixed | |||
| Acne keloidalis nuchae | Clobetasol 0.05% lotion or foamMonthly injections of triamcinolone acetonide 10 mg/mL | ClindamycinMinocycline or doxycycline100 mg/12-24 hCefradoxil 500 mg/12 h | Local protection measuresa) Incipient cases: potent topical corticosteroids +/- topical antibioticsb) Papular or papulonodular forms: injections of triamcinolone acetonide +/ - oral antibioticsc) Extensive keloids: surgery |
Dissecting folliculitis is a rare suppurative scalp disease with a chronic course found most commonly in black patients. It typically manifests in the form of suppurating follicular and perifollicular inflammatory nodules that give rise to interconnected sinus tracts resulting in scarring alopecia (Fig. 8). Under 10% of patients presenting with this disorder are white.67 In 30% of cases, dissecting folliculitis is associated with acne conglobata, hidradenitis suppurativa, and pilonidal cyst in what is known as the follicular occlusion tetrad. All of these conditions appear to be caused by an abnormal follicular keratinization that produces follicular obstruction, secondary bacterial infection, and destruction of the hair follicle.68 Usually located on the occipital or vertex region of the scalp, the first manifestation is a pustule that evolves rapidly to become a painful firm or fluctuant nodule. Subsequently, additional pustules and nodules appear in the adjacent area, giving the scalp a cerebriform appearance. The nodules suppurate spontaneously. The lesions evolve leaving atrophic scarring or keloidal alopecia.69 Occipital lymphadenopathy and an elevated serum erythrocyte sedimentation rate are often observed.
Prolonged remission has been achieved with isotretinoin, the first-line treatment.67 The recommended dose is 1mg/kg/d for 4 months, followed by 0.75mg/kg/d for a further 6 months. Some patients with intolerance to this regimen have responded equally well to continuous treatment with low doses of isotretinoin (10mg/d).70 Resistant cases have been reported to respond to combinations of oral rifampicin and isotretinoin71 and oral isotretinoin and dapsone.72 A single case of resistant disease successfully controlled with infliximab has been reported.73 Another case responded favorably to treatment with topical isotretinoin.74
Tufted FolliculitisTufted folliculitis is a unusual form of progressive and recurrent folliculitis that resolves leaving irregular patches of scarring alopecia.75
The etiology and pathogenesis of this condition are poorly understood and there is debate about whether it is in fact a separate nosologic entity.76 It is thought that it might be related to an inappropriate immune response to S aureus, since this pathogen has been isolated in most of these follicular lesions. Clinically, tufted folliculitis is characterized by tufts of hair growing on patches of scarring alopecia, giving the scalp a characteristic doll's head appearance.77
Histopathologic findings include a perifollicular inflammatory infiltrate located in the upper and mid-dermis. Hair detritus is also observed in the cytoplasm of macrophages and in multinucleate giant cells.76–78 This condition has usually been treated with topical and systemic antibiotics, especially antistaphylococcal agents; however, complete cure is rare. Powell et al54 used an antibiotic regimen combining rifampicin 300mg/12 h and clindamycin 300mg/12h for 10 weeks, which was effective in 10 out of 18 patients.54 Other treatments that have been used with limited success include oral isotretinoin, corticosteroids, and zinc sulfate. Surgery may be used to manage localized lesions that do not respond to medical treatment.
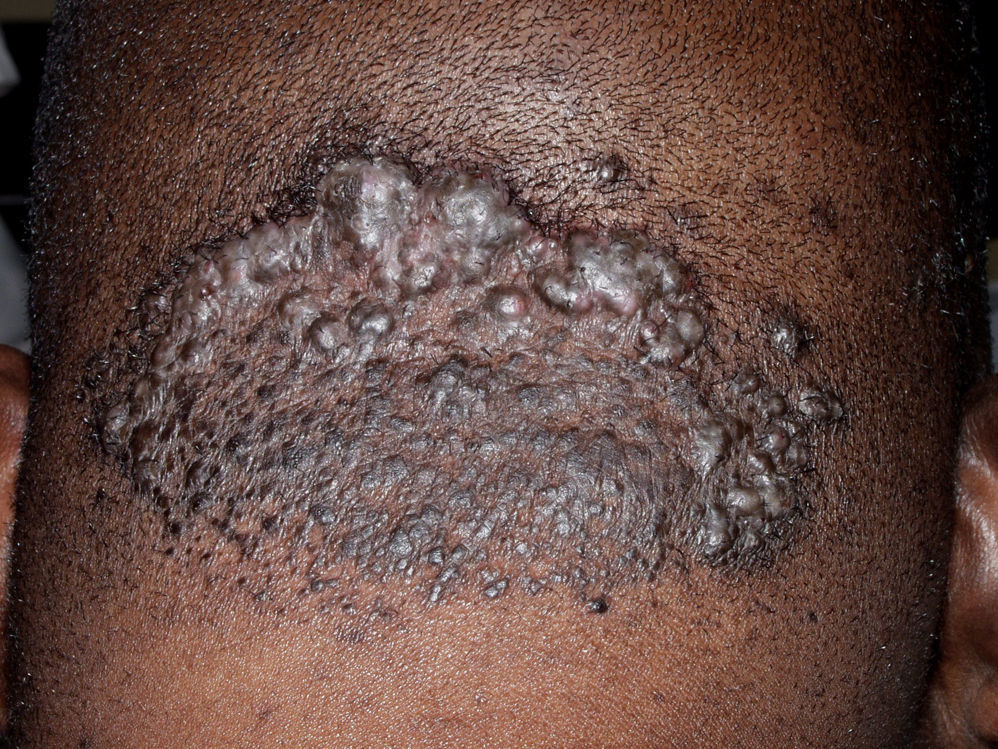
Mixed Primary Cicatricial AlopeciasAcne Keloidalis NuchaeAcne keloidalis nuchae is a chronic inflammatory process that mainly affects young black men. In some African countries the condition accounts for between 1.3% and 9.4% of dermatologic consultations in the hospital setting.79,80 It is a chronic scarring folliculitis that presents as follicular papulopustules, which rapidly progress to fibrotic papules and flesh-colored or hyperpigmented nodules (Fig. 9). The lesions are localized on the neck and occipital scalp. The crown and parietal region are rarely affected. Papules often coalesce to form keloidal plaques. This condition occasionally manifests as abscesses and sinus tracts with purulent discharge or tufted folliculitis.81 Precipitating factors include mechanical trauma (rubbing of a collar, close shaving or very short haircut, excoriations caused by scratching, the use of harsh chemical hair products,79 and seborrhea.
TreatmentEarly diagnosis is important in the treatment of acne keloidalis because it allows the patient to start aggressive treatment to reduce the risk of scarring, to minimize traumas (rubbing caused by the collars of shirts and t-shirts, shaving the scalp, and very short hair cuts), and to avoid exposure to the harsh chemical hair products used in some traditional remedies.79,80 Incipient disease can be treated with potent topical corticosteroids, such as clobetasol propionate 0.05% in lotion or foam either alone82 or in combination with topical antibiotics.83 The papular and papulonodular forms can be controlled with monthly injections of triamcinolone acetonide (10mg/mL) alone or in combination with oral antibiotics (clindamycin or tetracyclines such as minocycline or doxycycline 100mg/12-24h or cefradroxil 500mg/12h after the results of culture and sensitivity testing have been received) (Table 3). Rifampicin has also been shown to be effective, although this agent should never be used in single drug therapy.83,84 The use of antiseptic soaps such as chlorhexidine is also recommended as a complement to these treatments. There is no evidence that intralesional etanercept is more effective than injection of triamcinolone.85
Surgery is the treatment of choice in patients with very extensive keloidal plaques. Some experts have recommended treating keloidal papules with diode laser hair removal techniques once the bacterial infection has been cleared. This approach has resulted in a 90% to 95% improvement after 4 treatment sessions over 1 or 2 months with no new lesions during a 6-month follow-up84; however the usefulness of this treatment regimen is still a matter of debate.83
Acne Necrotic Varioliformis (Necrotizing Lymphocytic Folliculitis)Necrotic acne is a rare disorder of unknown etiology that affects adults and is associated with outbreaks of erythematous follicular papulopustules with central necrosis that resolve leaving a depressed scar.86 The areas most often affected are the nose, forehead, and anterior scalp.
The salient histopathologic features are a marked perivascular and perifollicular lymphocytic infiltrate extending into the mid-dermis associated with prominent subepidermal edema and necrosis of individual keratinocytes.86 The first rule in the management of this condition is to avoid all manipulation of the lesions. Antibiotic therapy should be prescribed according to the results of microbiological culture. Antistaphylococcal antibiotics such as cloxacillin or fusidic acid are the most common antibacterial therapy. However, good results have also been obtained with broad-spectrum antibiotics such as tetracyclines.87,88 In cases refractory to antibiotic treatment, oral isotretinoin has been used at a dose of 1 to 2mg/kg/d for 20 weeks.89 Potent topical corticosteroids and intralesional injections of triamcinolone acetonide have also been used with varying results.
Erosive Pustular DermatosisErosive pustular dermatosis is a rare skin disease of the scalp characterized by pustular lesions accompanied by erosive and crusted lesions that lead to scarring alopecia.90 The course is slow and progressive and the disease tends to be asymptomatic. The etiology and pathogenesis are poorly understood, although the condition is presumed to be related to local trauma, which is frequently reported in published cases.91 Prolonged exposure to ultraviolet radiation is another factor believed to be related to the development of this disease. Histopathologic findings are nonspecific and include epidermal atrophy and a predominantly lymphocytic mixed chronic inflammatory infiltrate in the dermis. It has been observed that antibiotics are only useful in the treatment of this condition when there is bacterial superinfection. The best results have been achieved with high-potency topical corticosteroids.92,93 However, relapse is common upon withdrawal of treatment and a maintenance treatment is therefore necessary. Good results have also been reported with topical tacrolimus,94 isotretinoin,95 acitretin,96 and oral zinc sulfate.97
Conflicts of InterestThe authors declare that they have no conflicts of interest.