Psoriatic arthritis (PsA) poses a diagnostic and therapeutic challenge. Timely initiation of advanced therapies can change the course of the disease and prevent its progression.1 Bimekizumab, an IgG1 antibody inhibiting both IL-17A and IL-17F, has proven effective in the management of psoriasis (Ps).2 However, despite robust phase III trial data (BE OPTIMAL, BE COMPLETE, and BE MOBILE 1–2),3–5 real-world evidence of its effectiveness in treating joint disease remains limited due to its recent approval. Therefore, we aimed to evaluate the short- and mid-term safety and efficacy profile of bimekizumab in treating patients with Ps and concomitant PsA across various Spanish hospitals.
We conducted a multicenter, non-interventional, retrospective study of Ps-PsA patients on bimekizumab at 11 hospitals from the Valencian Community, Spain from January 2023 to January 2024. Baseline characteristics, prior treatments, and comorbidities were reported. The dose of bimekizumab used in our study was the approved one to treat plaque Ps. Treatment response was analyzed in cutaneous and articular domains using the Psoriasis Area and Severity Index (PASI), Number of Tender Joints (NTJ), Number of Swollen Joints (NSJ), Physician's Global Assessment (PGA) for joint involvement, ACR50 and ACR75 response, and DLQI. Influence of various variables on joint response after 16 weeks were evaluated, along with safety profile.
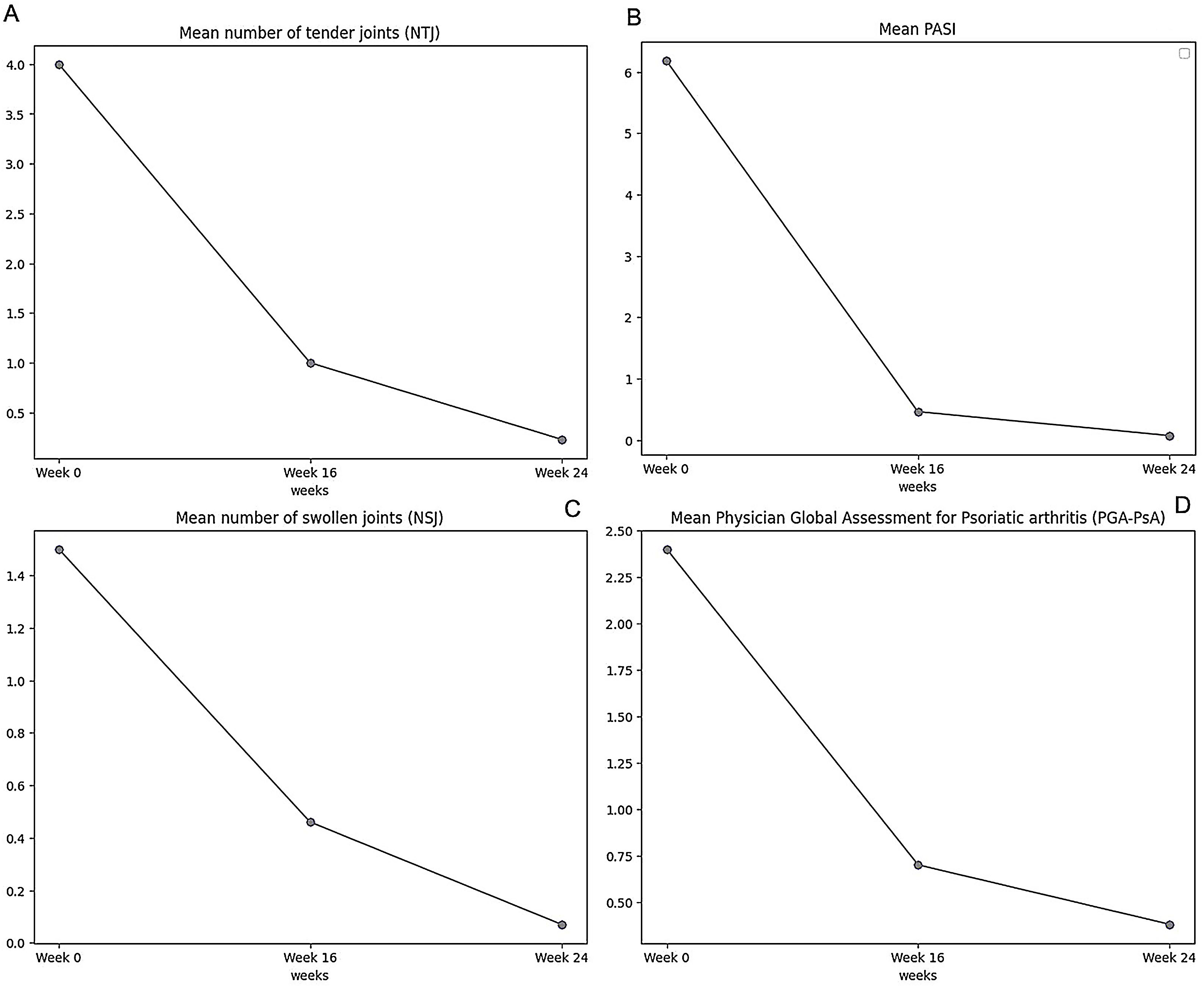
A total of 18 patients were included, with a mean age (SD) of 57.4 (12.6) years and a BMI (SD) of 28.1 (5.1) mean exposure (SD) to bimekizumab was 9.1 (3.9) months. Patients had a mean (SD) of 2.7 (2.2) prior biological therapies. Baseline cohort characteristics are detailed in Table 1. Rapid responses to bimekizumab were observed in skin and joints: the mean PASI score (SD) dropped from 6.2 (5.1) at baseline down to 0.5 (0.8) at week 16 and 0.1 (0.3) at week 24. DLQI (SD) improved significantly, dropping from 12.8 (5.7) at baseline down to 3.4 (3.9) at week 16. Joint response metrics (NTJ, NSJ, PGA) are shown in Fig. 1. ACR50 and ACR75 response were achieved by 77.7% and 50% of patients at week 16, respectively. Sex (p=0.658), presence of cardiovascular risk factors (p=0.522), and involvement of special areas (p=0.354) did not impact joint response at week 16. Linear regression analysis showed significant effects of PsA duration and prior biological therapies on PsA-PGA at week-16; BMI and baseline PASI were not statistically associated (Table of the supplementary data). The safety profile of bimekizumab was favorable, with no reports of serious infections or major adverse cardiovascular events (MACEs). Three patients experienced mild adverse events (2 candidiasis, 1 local injection site reaction).
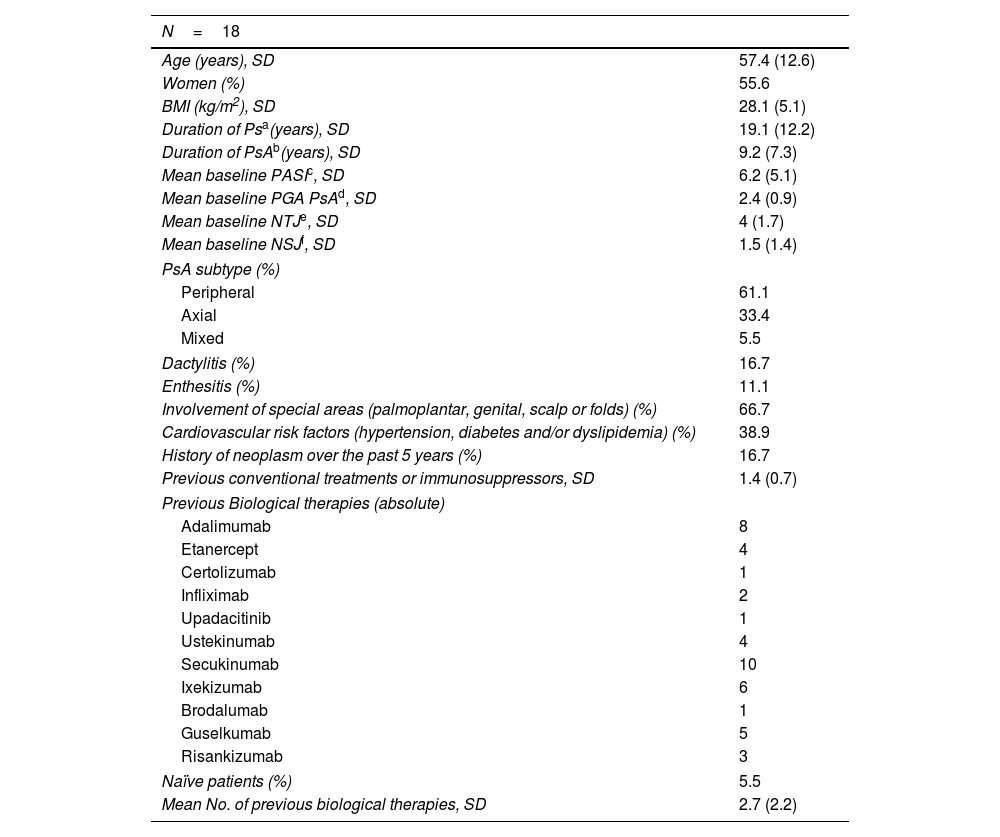
Baseline characteristics of the patients.
| N=18 | |
|---|---|
| Age (years), SD | 57.4 (12.6) |
| Women (%) | 55.6 |
| BMI (kg/m2), SD | 28.1 (5.1) |
| Duration of Psa(years), SD | 19.1 (12.2) |
| Duration of PsAb(years), SD | 9.2 (7.3) |
| Mean baseline PASIc, SD | 6.2 (5.1) |
| Mean baseline PGA PsAd, SD | 2.4 (0.9) |
| Mean baseline NTJe, SD | 4 (1.7) |
| Mean baseline NSJf, SD | 1.5 (1.4) |
| PsA subtype (%) | |
| Peripheral | 61.1 |
| Axial | 33.4 |
| Mixed | 5.5 |
| Dactylitis (%) | 16.7 |
| Enthesitis (%) | 11.1 |
| Involvement of special areas (palmoplantar, genital, scalp or folds) (%) | 66.7 |
| Cardiovascular risk factors (hypertension, diabetes and/or dyslipidemia) (%) | 38.9 |
| History of neoplasm over the past 5 years (%) | 16.7 |
| Previous conventional treatments or immunosuppressors, SD | 1.4 (0.7) |
| Previous Biological therapies (absolute) | |
| Adalimumab | 8 |
| Etanercept | 4 |
| Certolizumab | 1 |
| Infliximab | 2 |
| Upadacitinib | 1 |
| Ustekinumab | 4 |
| Secukinumab | 10 |
| Ixekizumab | 6 |
| Brodalumab | 1 |
| Guselkumab | 5 |
| Risankizumab | 3 |
| Naïve patients (%) | 5.5 |
| Mean No. of previous biological therapies, SD | 2.7 (2.2) |
To date, real-world-evidence on the joint response of bimekizumab is limited. Our findings demonstrate superior cutaneous and joint outcomes vs clinical trials,3–5 despite the high proportion of patients with previously failed biological therapies. Of note the relationship between disease duration, prior biologics, and likelihood of achieving optimal joint response by week 16, underscoring the importance of early intervention. In conclusion, bimekizumab emerges as a viable therapeutic option for PsA, offering substantial clinical benefits and a manageable safety profile in real-world setting. Further studies are warranted to substantiate these findings and optimize PsA management strategies.
Authors’ contributionsF.J. Melgosa Ramos has contributed to the conception, design, data collection, interpretation, writing-draft preparation and review of the present work. M. Mansilla Polo, J.M. Ortiz Salvador and A. Martorell have contributed to data collection. The final version to be published has been approved by all the authors.
Ethics statementThe study was conducted in accordance with the principles contained in the Declaration of Helsinki for studies involving humans. All patients provided their informed written consent.
Funding informationNo payments were made to the authors for the development of this manuscript.
Conflict of interestThe author(s) declare(s) that there are no conflicts of interest regarding the publication of this paper.
Data availabilityAll data are available under request from the authors.
The patient in this manuscript has given informed consent to the publication of their case details.