Rituximab is a chimeric mouse-human antibody that targets the CD20 antigen, which is found in both normal and neoplastic B cells. In recent years, it has been increasingly used to treat cutaneous B-cell lymphoma and is now considered an alternative to classic treatment (radiotherapy and surgery) of 2 types of indolent lymphoma, namely, primary cutaneous follicle center lymphoma and primary cutaneous marginal zone B-cell lymphoma. Rituximab is also administered as an alternative to polychemotherapy in the treatment of primary cutaneous large B-cell lymphoma, leg type. Its use as an alternative drug led to it being administered intralesionally, with beneficial effects. In the present article, we review the literature published on the use of rituximab to treat primary cutaneous B-cell lymphoma.
Rituximab es un anticuerpo quimérico murino-humano dirigido contra el antígeno CD20 presente en los linfocitos B normales y neoplásicos. Su uso en los linfomas cutáneos de células B ha ido en creciente desarrollo en los últimos años. Así se plantea como una alternativa a los tratamientos clásicos de radioterapia y cirugía en los linfomas de curso indolente, el linfoma folicular y el linfoma de la zona marginal. También se utiliza en el tratamiento del linfoma cutáneo primario de células grandes tipo piernas como alternativa a la poliquimioterapia. Su desarrollo como alternativa terapéutica ha llevado a su uso intralesional también con buenos resultados. En este artículo se revisa la literatura publicada del uso de rituximab en los linfomas cutáneos primarios de células B.
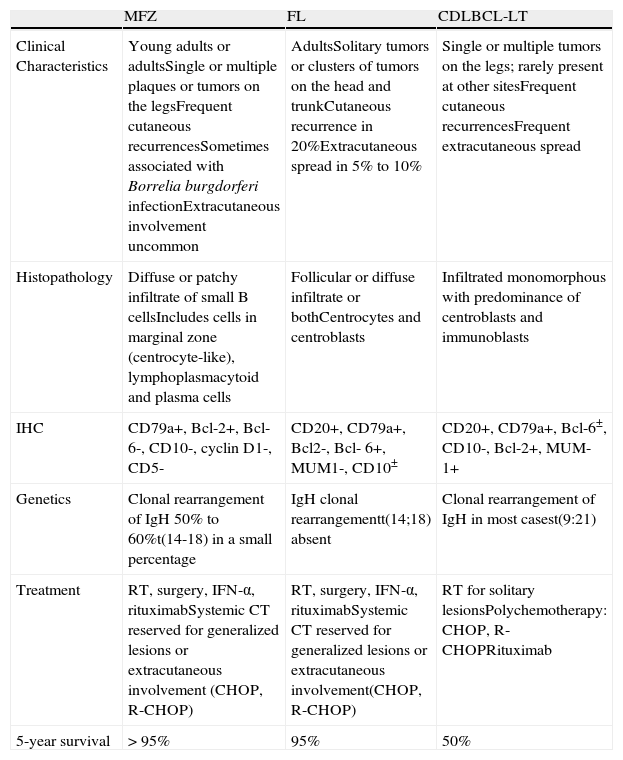
Primary cutaneous B-cell lymphomas (CBCLs) are a group of B-cell lymphomas localized in the skin at the time of diagnosis. According to the recent World Health Organization (WHO)-European Organization for Research and Treatment of Cancer (EORTC) classification, there are 3 main subgroups: follicular lymphoma (FL), marginal zone lymphoma (MZL), and cutaneous diffuse large B-cell lymphoma leg-type (CDLBCL-LT).1 These account for approximately 20% to 25% of all primary cutaneous lymphomas. Most CBLCs (more than 80%) correspond to the 2 variants with an indolent course, FL and MZL.2 The main characteristics of these 3 subgroups are summarized in Table 1.3 Neither FL nor MZL follows a very aggressive course, and 5-year survival is greater than 95%.1 Although prognosis is excellent, recurrence rates after treatment are high, ranging from 14% to 62%.3 FL and MZL most frequently present as plaque or nodular lesions, occasionally with an anatomic distribution, with the trunk being the most frequent site (48.9%), followed by the face (26.4%).4,5 Given the benign course of these cutaneous B-cell lymphomas and their frequent multifocal distribution, a conservative approach to treatment should be followed. The most widely used types of treatment are radiotherapy (RT) and surgery,4,5 though these carry with them a risk of sequelae and are not ideal in the case of multifocal and/or recurrent lesions. Other therapeutic options therefore need to be explored. In absence of controlled clinical trials, there is no clear consensus on the best treatment for indolent CBCL.3 CDLBCL-LT in contrast follows a rapidly progressing course with a high rate of recurrence and a tendency for extracutaneous dissemination; 5-year survival is 50%.3 The lesions are located on the legs in more than 70% of patients, with presentation in the form of nodules or ulcerated tumors. Differential diagnosis should include systemic diffuse large cell non-Hodgkin lymphoma (NHL) with cutaneous involvement.6 In recent years, systemic rituximab alone or in combination with chemotherapeutic agents has been introduced for the treatment of CDLBCL-LT.
Characteristics of the Most Common Primary Cutaneous B-Cell Lymphomas.
| MFZ | FL | CDLBCL-LT | |
| Clinical Characteristics | Young adults or adultsSingle or multiple plaques or tumors on the legsFrequent cutaneous recurrencesSometimes associated with Borrelia burgdorferi infectionExtracutaneous involvement uncommon | AdultsSolitary tumors or clusters of tumors on the head and trunkCutaneous recurrence in 20%Extracutaneous spread in 5% to 10% | Single or multiple tumors on the legs; rarely present at other sitesFrequent cutaneous recurrencesFrequent extracutaneous spread |
| Histopathology | Diffuse or patchy infiltrate of small B cellsIncludes cells in marginal zone (centrocyte-like), lymphoplasmacytoid and plasma cells | Follicular or diffuse infiltrate or bothCentrocytes and centroblasts | Infiltrated monomorphous with predominance of centroblasts and immunoblasts |
| IHC | CD79a+, Bcl-2+, Bcl-6-, CD10-, cyclin D1-, CD5- | CD20+, CD79a+, Bcl2-, Bcl- 6+, MUM1-, CD10± | CD20+, CD79a+, Bcl-6±, CD10-, Bcl-2+, MUM-1+ |
| Genetics | Clonal rearrangement of IgH 50% to 60%t(14-18) in a small percentage | IgH clonal rearrangementt(14;18) absent | Clonal rearrangement of IgH in most casest(9:21) |
| Treatment | RT, surgery, IFN-α, rituximabSystemic CT reserved for generalized lesions or extracutaneous involvement (CHOP, R-CHOP) | RT, surgery, IFN-α, rituximabSystemic CT reserved for generalized lesions or extracutaneous involvement(CHOP, R-CHOP) | RT for solitary lesionsPolychemotherapy: CHOP, R-CHOPRituximab |
| 5-year survival | >95% | 95% | 50% |
Rituximab is a murine-human chimeric monoclonal immunoglobulin G antibody against the CD20 antigen present on almost all neoplastic and normal B-cells. It has been used successfully in the treatment of systemic B-cell NHL either as monotherapy or more commonly in combination with other chemotherapeutic agents.7 In recent years, rituximab has been used by dermatologists with good outcomes in a range of skin diseases8 and CBCLs.
In vitro studies of rituximab have shown this agent induces lysis of lymphoma B-cells by antibody-dependent cell mediated cytotoxicity, complement activation, and direct induction of apoptosis. This mechanism does not depend on the immune system and occurs because the variable region of murine origin binds with high affinity to the CD20 antigen expressed on malignant B lymphocytes, halting their proliferation and inducing apoptosis probably through transmembrane calcium channels.6 Rituximab has also been shown to induce an antigen-specific response in T cells by an immunization mechanism.9 Additionally the drug is able to sensitize cells to the cytotoxic effects of other chemotherapeutic agents.10
Rituximab is also known to act via the bcl-2 protein. This marker is overexpressed in 85% of CDLBCL-LT and is considered one of the factors associated with worse prognosis.11 In the case of systemic diffuse large cell lymphomas, the addition of rituximab to combinations based on anthracyclines is particularly beneficial for bcl-2 positive lymphomas, which had a worse prognosis before rituximab was available.12
Rituximab in Primary Cutaneous LymphomasFollicular Lymphoma and Marginal Zone LymphomaPrimary cutaneous FL and MZL are cutaneous lymphomas with an indolent course despite the high rates of recurrence. It is therefore important to highlight that treatment should not be aggressive in most cases. The recommendations for management of LF and MZL include withholding treatment (watchful waiting), RT, surgery, or intralesional interferon alfa.3 Sometimes, however, large, painful, disfiguring, or itchy multiple lesions may be present; watchful waiting is not an option and treatment should be administered. Moreover, in these situations, local skin interventions, such as surgery or RT, are not appropriate, particularly in the case of multiple lesions or lesions on sites such as the face or neck where the interventions might leave substantial esthetic sequelae. In these circumstances, other treatment alternatives, among them systemic or intralesional rituximab, have been used.
Tables 2 and 3 summarize the published cases of FL and MZL, respectively, treated with systemic rituximab. Table 4 summarizes the cases of FL and MZL treated with intralesional administration of rituximab.
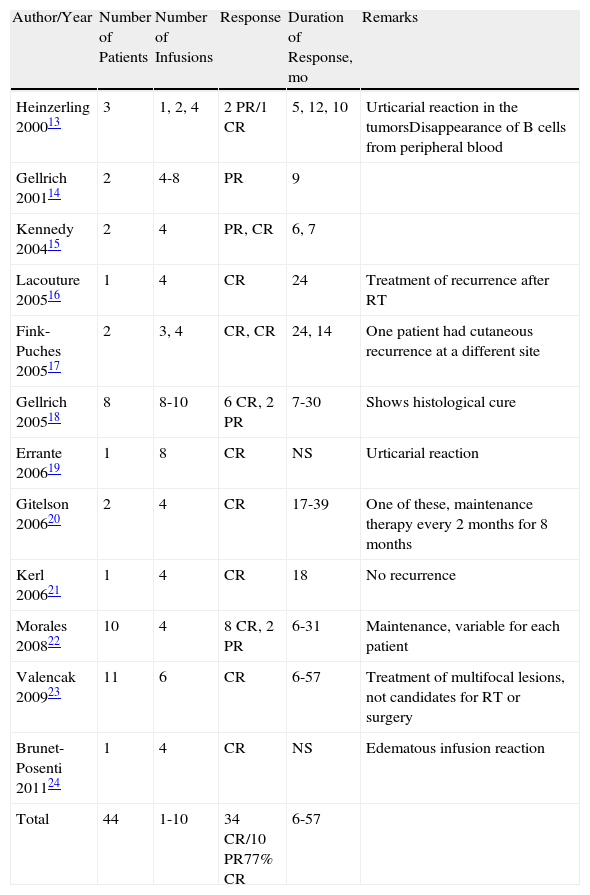
Published Cases of Follicular Lymphoma Treated with Intravenous Rituximab.
| Author/Year | Number of Patients | Number of Infusions | Response | Duration of Response, mo | Remarks |
| Heinzerling 200013 | 3 | 1, 2, 4 | 2 PR/1 CR | 5, 12, 10 | Urticarial reaction in the tumorsDisappearance of B cells from peripheral blood |
| Gellrich 200114 | 2 | 4-8 | PR | 9 | |
| Kennedy 200415 | 2 | 4 | PR, CR | 6, 7 | |
| Lacouture 200516 | 1 | 4 | CR | 24 | Treatment of recurrence after RT |
| Fink-Puches 200517 | 2 | 3, 4 | CR, CR | 24, 14 | One patient had cutaneous recurrence at a different site |
| Gellrich 200518 | 8 | 8-10 | 6 CR, 2 PR | 7-30 | Shows histological cure |
| Errante 200619 | 1 | 8 | CR | NS | Urticarial reaction |
| Gitelson 200620 | 2 | 4 | CR | 17-39 | One of these, maintenance therapy every 2 months for 8 months |
| Kerl 200621 | 1 | 4 | CR | 18 | No recurrence |
| Morales 200822 | 10 | 4 | 8 CR, 2 PR | 6-31 | Maintenance, variable for each patient |
| Valencak 200923 | 11 | 6 | CR | 6-57 | Treatment of multifocal lesions, not candidates for RT or surgery |
| Brunet-Posenti 201124 | 1 | 4 | CR | NS | Edematous infusion reaction |
| Total | 44 | 1-10 | 34 CR/10 PR77% CR | 6-57 |
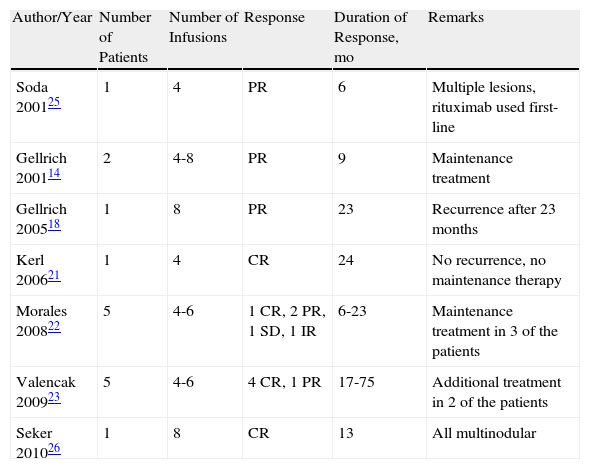
Published Cases of Marginal Zone Lymphoma Treated with Intravenous Rituximab.
| Author/Year | Number of Patients | Number of Infusions | Response | Duration of Response, mo | Remarks |
| Soda 200125 | 1 | 4 | PR | 6 | Multiple lesions, rituximab used first-line |
| Gellrich 200114 | 2 | 4-8 | PR | 9 | Maintenance treatment |
| Gellrich 200518 | 1 | 8 | PR | 23 | Recurrence after 23 months |
| Kerl 200621 | 1 | 4 | CR | 24 | No recurrence, no maintenance therapy |
| Morales 200822 | 5 | 4-6 | 1 CR, 2 PR, 1 SD, 1 IR | 6-23 | Maintenance treatment in 3 of the patients |
| Valencak 200923 | 5 | 4-6 | 4 CR, 1 PR | 17-75 | Additional treatment in 2 of the patients |
| Seker 201026 | 1 | 8 | CR | 13 | All multinodular |
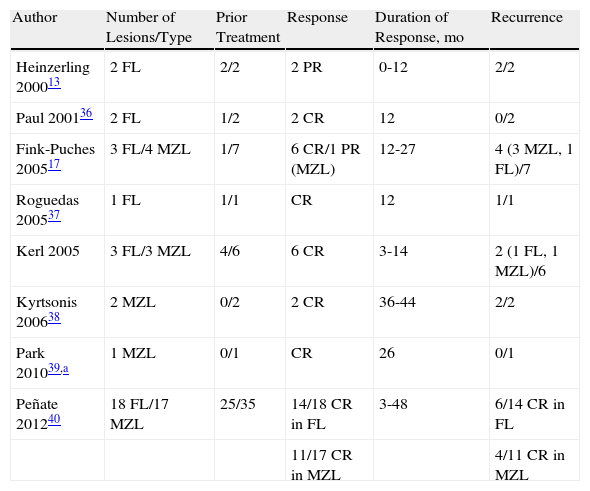
Published Cases of Follicular Lymphoma Treated with Intralesional Rituximab.
| Author | Number of Lesions/Type | Prior Treatment | Response | Duration of Response, mo | Recurrence |
| Heinzerling 200013 | 2 FL | 2/2 | 2 PR | 0-12 | 2/2 |
| Paul 200136 | 2 FL | 1/2 | 2 CR | 12 | 0/2 |
| Fink-Puches 200517 | 3 FL/4 MZL | 1/7 | 6 CR/1 PR (MZL) | 12-27 | 4 (3 MZL, 1 FL)/7 |
| Roguedas 200537 | 1 FL | 1/1 | CR | 12 | 1/1 |
| Kerl 2005 | 3 FL/3 MZL | 4/6 | 6 CR | 3-14 | 2 (1 FL, 1 MZL)/6 |
| Kyrtsonis 200638 | 2 MZL | 0/2 | 2 CR | 36-44 | 2/2 |
| Park 201039,a | 1 MZL | 0/1 | CR | 26 | 0/1 |
| Peñate 201240 | 18 FL/17 MZL | 25/35 | 14/18 CR in FL | 3-48 | 6/14 CR in FL |
| 11/17 CR in MZL | 4/11 CR in MZL |
In total, 44 cases have been published of cutaneous FL treated with intravenous rituximab (Table 2).13–24 Regimens have ranged from the usual 4 infusions, once a week, with standard doses of 375mg/m2 to courses of up to 10 infusions. The complete response rate for all the published cases is approximately 77%, with a response duration ranging from 6 to 57 months. For MZL, 16 cases of systemic rituximab treatment have been published (Table 3),14,18,21,25,26 with an overall complete response rate of 43% and a duration of response of between 6 and 75 months.
This apparently worse response to rituximab in patients with MZL is pending confirmation in the future in large prospective studies; if real, the difference could be due to several mechanisms that confer resistance to rituximab. A study has been published describing the lack of efficacy of rituximab in patients with noncutaneous MZL who also received chemoimmunotherapy.27 One of the hypotheses is that malignant B cells acquire new mutations that confer resistance to rituximab-induced apoptosis. Thus, in a study of 4 patients with recurrent disease, an increase in bcl-2 activity was observed after rituximab treatment.28 Variations in the site of B-cell binding to rituximab have also been seen. Patients with certain genotypes at the antibody binding site appear to respond better to treatment with rituximab and disease-free periods are longer. This polymorphism in the expression of the CD20 antigen in malignant B cells may define a subgroup of patients with innate resistance to rituximab.29
Recurrences after treatment with intravenous rituximab, in both patients with FL and with MZL, are frequent (20% and 50%, respectively). However, recurrences of CBCL are frequent with all treatment types except surgery and RT.3 Thus, authors such as Gellrich et al.18 propose more prolonged treatments of 8 cycles instead of 4, as better response rates (90%) and remission rates are obtained; however, the benefits of prolonging treatment are not clearly demonstrated due to the small number of patients studied. In a subsequent study of 16 patients, Valencak et al.23 reported complete response in 62% of indolent CBCL treated with intravenous rituximab and proposed that 4 cycles would be sufficient. The authors highlighted the lack of studies that support the need to extend treatment. Likewise, the published studies do not provide clear indications as to whether re-treatment with rituximab after recurrence might be beneficial, as has been demonstrated in systemic FL.30,31
Most studies define complete response as disappearance of all cutaneous lesions, which are assessed clinically with regular follow-up of the patients. Some studies have, however, also evaluated histologic response of the lesions14,18,25 by means of a skin biopsy after treatment. These studies report the presence of an infiltrate of CD8-positive T cells,14 with decreased CD20 expression.25 Gellrich et al.18 took biopsies from 8 of the treated patients with complete response and found that despite a complete clinical response, only 6 had complete histologic response.
Intravenous treatment with rituximab is safe and generally well tolerated, particularly in comparison with other traditional chemotherapeutic agents. The adverse effects are summarized in Table 5.32 The most frequently reported cutaneous effects are mild, usually in the form of rash or urticaria, which occur in approximately 15% of patients. Serious side effects such as paraneoplastic pemphigus or Steven-Johnson syndrome appear in fewer than 2%. Of interest is the occurrence of wheals around the skin lesions in FL treated with systemic rituximab; this is thought to be due to the release of inflammatory cytokines.13,19 The risk of reactivation of hepatitis B virus is also of particular interest in patients with chronic infection as the event can potentially be fatal.26,33 The American Hepatology Guidelines recommend the use of lamivudine as prophylaxis for up to 6 months after finishing treatment with intravenous rituximab in carriers of HbsAg.34 Some authors suggest this prophylactic period should be extended to up to 2 years after finishing treatment, given that late reactivation of hepatitis B has been reported.26
Adverse Effects of Systemic Rituximab.
| Cutaneous Effects | RashPruritusUrticariaBacterial infectionsLichenoid reactionsParaneoplastic pemphigusSteven-Johnson syndrome/toxic epidermal necrosisVesicular/blistering dermatitis |
| Systemic Effects | Fatal infusion reaction (first 24h)Tumor lysis syndromeHepatitis B reactivation (can be fulminant)Severe cardiac arrhythmiasRenal failure (can be fatal)Hypersensitivity reactionsOthers: sick serum disease, rheumatoid-like inflammatory arthritis syndrome, vasculitis, mucositis |
Source: Scheinfield32
In most studies, intravenous rituximab is considered the treatment of choice for multifocal lesions and also for lesions on the face and scalp, as RT may cause alopecia and irreversible cutaneous effects, such as poichyloderma or atrophy.19,26
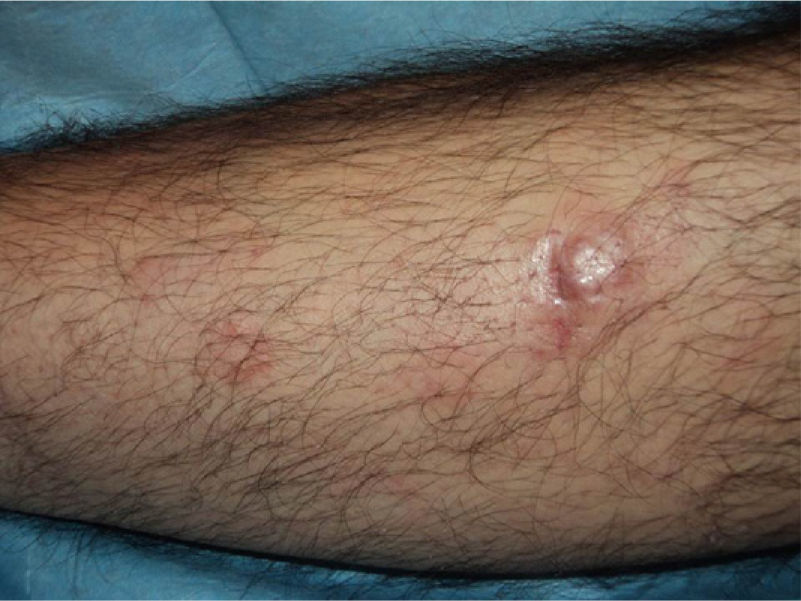
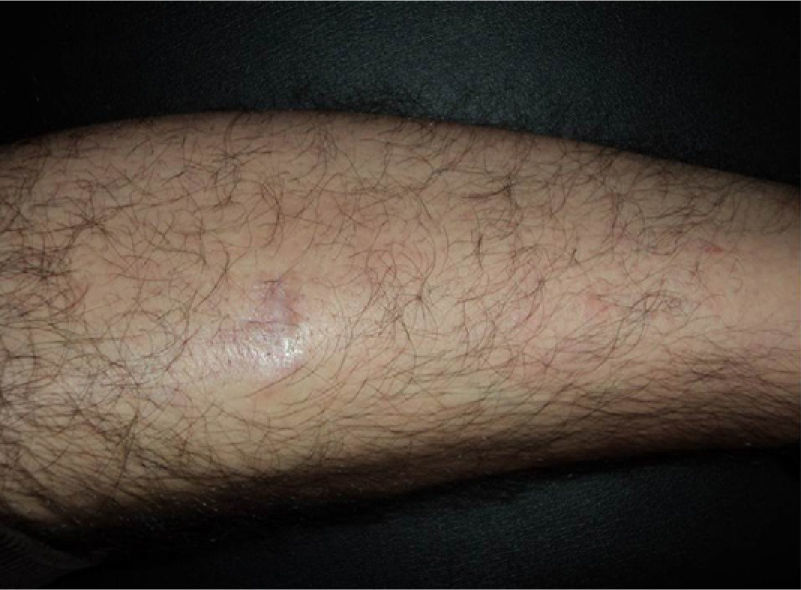
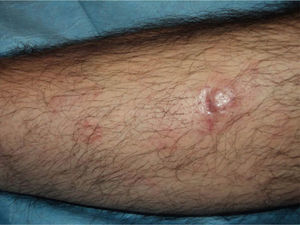
In recent years, the use of intralesional rituximab has become widespread for FL and MZL. The aim is to make treatment more comfortable and accessible while minimizing side effects (Figs. 1 and 2). The published cases of treatment of FL and MZL with intralesional rituximab are summarized in Table 6.17,21,35–40 The complete response rates with this treatment in indolent B-cell lymphomas are somewhat greater than those achieved with intravenous administration. Response rates range from 83% to 89%, but the recurrence rate after treatment remains high (40% to 62%).3 In most studies, regimens of between 10 and 30mg per lesion (diluted to 10mg/mL) are used, up to 3 times a week and in cycles of up to 12 weeks, according to response. The most frequently reported side effect is injection-site pain.17,21,35–39 Of particular note given the large number of patients included is a recent study published by the Spanish Cutaneous Lymphoma Group, who retrospectively analyzed the outcomes of intralesional rituximab treatment of indolent cutaneous B-cell lymphoma in several hospitals.40 A total of 35 patients were included, 17 with MZL and 18 with FL. A mean of 2 lesions were treated per patient. Most patients were treated with 3 injections per week for 1 week in every month. A complete response rate of 74% was found—slightly below that reported in previous studies.3 Mean disease-free survival was 114 weeks. Side effects were detected in 54% of the patients, with the most common being injection-site pain. A small number of patients had fever presenting between 6 and 12 hours after the injection, although the effect was transient and observed most often in the first injections. Some of the most important findings in this study are that there were no apparent factors predictive of response or differences in responses according to underlying disease (MZL or FL), in contrast to the case when the drug is administered systemically.
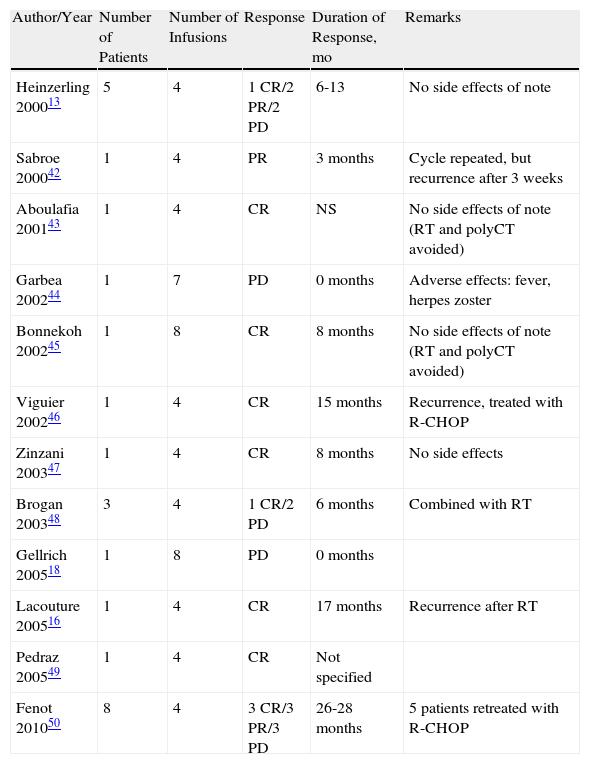
Cutaneous Diffuse Large B-Cell Lymphoma Leg-Type, Treated With Rituximab Monotherapy.
| Author/Year | Number of Patients | Number of Infusions | Response | Duration of Response, mo | Remarks |
| Heinzerling 200013 | 5 | 4 | 1 CR/2 PR/2 PD | 6-13 | No side effects of note |
| Sabroe 200042 | 1 | 4 | PR | 3 months | Cycle repeated, but recurrence after 3 weeks |
| Aboulafia 200143 | 1 | 4 | CR | NS | No side effects of note (RT and polyCT avoided) |
| Garbea 200244 | 1 | 7 | PD | 0 months | Adverse effects: fever, herpes zoster |
| Bonnekoh 200245 | 1 | 8 | CR | 8 months | No side effects of note (RT and polyCT avoided) |
| Viguier 200246 | 1 | 4 | CR | 15 months | Recurrence, treated with R-CHOP |
| Zinzani 200347 | 1 | 4 | CR | 8 months | No side effects |
| Brogan 200348 | 3 | 4 | 1 CR/2 PD | 6 months | Combined with RT |
| Gellrich 200518 | 1 | 8 | PD | 0 months | |
| Lacouture 200516 | 1 | 4 | CR | 17 months | Recurrence after RT |
| Pedraz 200549 | 1 | 4 | CR | Not specified | |
| Fenot 201050 | 8 | 4 | 3 CR/3 PR/3 PD | 26-28 months | 5 patients retreated with R-CHOP |
Source: Lacouture et al.16; Gellrich et al.18; Heinzerling et al.35; Sabroe et al.42; Aboulafia43; Garbea et al.44; Bonnekoh et al.45; Viguier et al.46; Zinzani et al.47; Brogan et al.48; Pedraz et al.49; and Fenot et al.50
Abbreviations: CR, complete response; CT, chemotherapy; NS, not specified; PD, progressive disease; PR, partial response; RT, radiotherapy; SD, stable disease
The conclusion from these studies is that intralesional use of rituximab is becoming more widespread in selected patients with low-grade CBCL in view of the convenience, lower dose, and fewer side effects.
Cutaneous Diffuse Large B-cell Lymphoma, Leg-TypeCDLBCL-LT occurs less frequently than the other 2 types of CBCL discussed above. This type accounts for approximately 1% to 3% of all primary cutaneous lymphomas and, unlike FL and MZL, follows a more aggressive course with frequent extracutaneous spread and recurrence after treatment. Prognosis is intermediate with a 5-year survival rate of approximately 50%.3 Factors indicative of poor prognosis in cutaneous large B-cell lymphomas include onset at an early age, ulceration, and positive staining for multiple myeloma oncogene (MUM) 1 and bcl-2, while expression of bcl-6 is associated with good prognosis.41 However, these findings are open to debate because the series of cutaneous large B-cell lymphomas include some forms of FL, which is typically bcl-6 positive and has a better prognosis. In contrast, CDLBCL-LT is typically MUM-1 positive and has a worse prognosis. The expression of one of these proteins may not therefore be a prognostic factor in itself, but rather related to diagnosis of 2 different lymphomas with different prognoses.
The guidelines for management of CBCL, issued by the EORTC, recommend treatment of CDLBCL-LT with polychemotherapy with or without rituximab (cyclophosphamide, hydroxydaunorubicin, Oncovin, prednisone [CHOP]-like or R-CHOP regimens). In localized disease, the recommended treatment is RT, which shows good response rates.3 There are 25 cases in the literature of CDLBCL-LT treated with rituximab in monotherapy (summarized in Table 6).13,16,42–50 Regimens of 4 to 8 infusions were used with or without local RT in the tumors. The complete response rate of the published cases is 48%, but this response is only maintained for more than 6 months in 4 of the patients, that is, 18%, and so recurrences after treatment are frequent (82%). In all cases, treatment was well tolerated, without significant side effects.
The role of rituximab as monotherapy in CDLBCL-LT is not well defined; the results reported in the literature suggest that there is a marked initial response but recurrences and disease progression are frequent.
The recommended treatment with CHOP or CHOP-like polychemotherapy in CDLBCL-LT obtains complete response rates of 81%, but once again recurrence rates are high at 54%.3 Some authors therefore recommend the use of intravenous rituximab as a less aggressive option that is better adapted to older patients.16,43,45,49 Despite the recommendations of the WHO-EORTC, some authors have wondered whether intensified treatments with greater toxicity really improve prognosis in these patients. Most suggest that the treatment of choice should be tailored and adapted to the age of the patient, with rituximab considered as a palliative measure.
Other B-Cell Lymphoproliferative ProcessesThere has been a report of a case of cutaneous intravascular large B-cell lymphoma treated with R-CHOP with complete clinical and histologic response maintained for 6 months. These results appear promising, as this is a very aggressive lymphoma. However, given the rarity of the condition, it is difficult to establish treatment protocols.51
There is also a published case of pseudo-B-cell lymphoma refractory to conventional therapies that responded well to intralesional rituximab.52
ConclusionsRituximab is an anti-CD20 antibody that is increasingly used in the treatment of CBCL. Good response rates are obtained in indolent B-cell lymphomas, FL, and MZL, for both the intravenous and intralesional route of administration, although recurrence is frequent. Rituximab is not the preferred treatment, but it should be considered in patients with multiple and/or recurrent lesions at visible sites where radiotherapy or surgery may leave sequelae or scars. Intralesional use has become widespread in recent years with similar outcomes to intravenous use, but with fewer side effects and a lower cost. More studies are needed to establish the usefulness and the optimal protocol in other B-cell cutaneous lymphomas. In the future, molecular predictors of response, new clinical trials, and new combinations with existing treatments or other monoclonal antibodies may help define the role of rituximab in the treatment of CBCL.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that patient data do not appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fernández-Guarino M, Ortiz-Romero PL, Fernández-Misa R, Montalbán C. Rituximab en el tratamiento de los linfomas cutáneos B primarios: revisión. Actas Dermosifiliogr. 2014;105:438–445.