Porokeratosis describes a heterogeneous group of keratinization disorders of unknown pathogenesis. It has various clinical forms that differ according to lesion number, size, and distribution. These include porokeratosis of Mibelli, disseminated superficial actinic porokeratosis, porokeratosis palmaris et plantaris disseminata, and linear porokeratosis.1 Lesions typically manifest as erythematous macules surrounded by a characteristically hyperkeratotic border. The most characteristic histologic findings are foci of dyskeratotic cells in the granular layer and columns of parakeratosis known as cornoid lamellae.1
Porokeratosis usually follows a chronic course and is refractory to treatment. A range of topical, systemic and physical treatments have been used with variable results.
Germline loss-of-function mutations in the HMG-CoA reductase pathway (specifically involving the phosphomevalonate kinase and mevalonate diphosphate decarboxylase genes) were recently described in porokeratosis. These mutations would lead to a deficiency in end products of this pathway (which include cholesterol) and subsequent accumulation of toxic metabolites and increased premature apoptosis of keratinocytes. The suggestion that these events might explain the porokeratosis phenotype2 has prompted the search for treatment alternatives specifically targeting the HMG-CoA reductase pathway. These alternatives include compounded cream or lotion formulations applied every 12 hours that combine cholesterol (generally at 2% or 5%) and a 2% statin (simvastatin or lovastatin). Current understanding of the pharmacokinetics of topically applied statins is limited, but it is thought that lipophilic statins (lovastatin, simvastatin) may be more effective due to greater transdermal absorption and that oral formulations are likely to be ineffective due to first-pass metabolism in the liver. It is also unclear whether treatment effectiveness is affected by specific mutations. Topical cholesterol appears to be less effective when administered as monotherapy. The combined treatment, by contrast, has the advantage of preventing the accumulation of toxic metabolites through the lowering of mevalonate levels.2,3
The reasoning behind the use of topical cholesterol/statin formulations for porokeratosis is linked to the favorable outcomes observed in CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and leg defects), where it is thought that skin lesions may result from the accumulation of toxic metabolites from the cholesterol pathway due to mutations in the NSDHL gene [Xq28], which codes for a protein involved in cholesterol biosynthesis.4
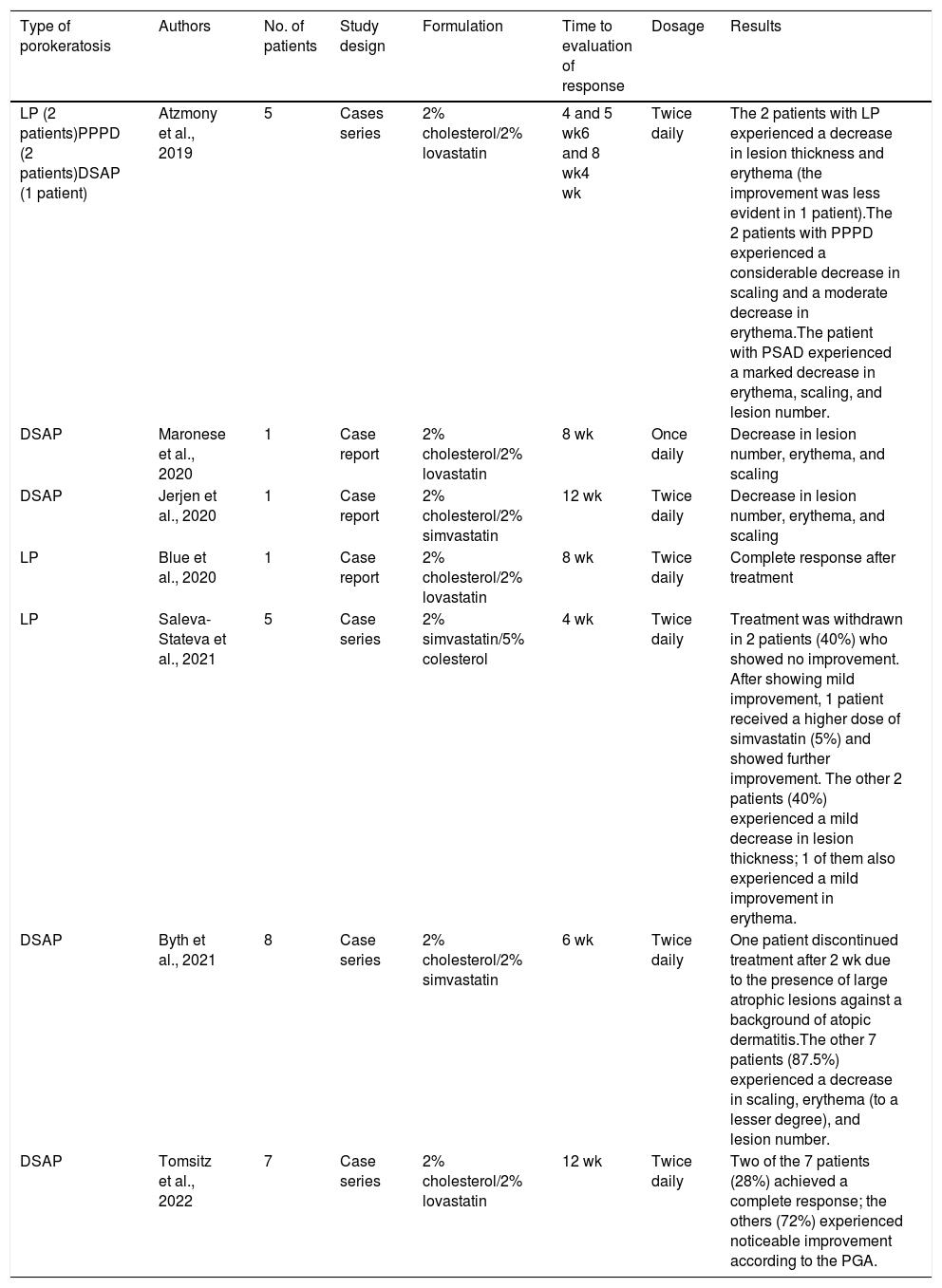
Case reports and series published since 2019 have reported favorable results for the use of topical cholesterol combined with either simvastatin or lovastatin in patients with porokeratosis (Table 1). The advantages of these formulations are their price, versatility (vehicles can be changed or adjuvants such as keratolytics added), and dosage (which can be maintained indefinitely).3,5 Although these novel formulations are being increasingly used in routine clinical practice, many cases are not reported. We believe it important to summarize the experience to date to consolidate existing knowledge and inform subsequent use.
Summary of Case Reports and Series Describing the Use of Topical Statin and Cholesterol Formulations in Porokeratosis.
| Type of porokeratosis | Authors | No. of patients | Study design | Formulation | Time to evaluation of response | Dosage | Results |
|---|---|---|---|---|---|---|---|
| LP (2 patients)PPPD (2 patients)DSAP (1 patient) | Atzmony et al., 2019 | 5 | Cases series | 2% cholesterol/2% lovastatin | 4 and 5 wk6 and 8 wk4 wk | Twice daily | The 2 patients with LP experienced a decrease in lesion thickness and erythema (the improvement was less evident in 1 patient).The 2 patients with PPPD experienced a considerable decrease in scaling and a moderate decrease in erythema.The patient with PSAD experienced a marked decrease in erythema, scaling, and lesion number. |
| DSAP | Maronese et al., 2020 | 1 | Case report | 2% cholesterol/2% lovastatin | 8 wk | Once daily | Decrease in lesion number, erythema, and scaling |
| DSAP | Jerjen et al., 2020 | 1 | Case report | 2% cholesterol/2% simvastatin | 12 wk | Twice daily | Decrease in lesion number, erythema, and scaling |
| LP | Blue et al., 2020 | 1 | Case report | 2% cholesterol/2% lovastatin | 8 wk | Twice daily | Complete response after treatment |
| LP | Saleva-Stateva et al., 2021 | 5 | Case series | 2% simvastatin/5% colesterol | 4 wk | Twice daily | Treatment was withdrawn in 2 patients (40%) who showed no improvement. After showing mild improvement, 1 patient received a higher dose of simvastatin (5%) and showed further improvement. The other 2 patients (40%) experienced a mild decrease in lesion thickness; 1 of them also experienced a mild improvement in erythema. |
| DSAP | Byth et al., 2021 | 8 | Case series | 2% cholesterol/2% simvastatin | 6 wk | Twice daily | One patient discontinued treatment after 2 wk due to the presence of large atrophic lesions against a background of atopic dermatitis.The other 7 patients (87.5%) experienced a decrease in scaling, erythema (to a lesser degree), and lesion number. |
| DSAP | Tomsitz et al., 2022 | 7 | Case series | 2% cholesterol/2% lovastatin | 12 wk | Twice daily | Two of the 7 patients (28%) achieved a complete response; the others (72%) experienced noticeable improvement according to the PGA. |
Abbreviations: LP, linear porokeratosis; PGA, Physician's Global Assessment; PPPD, porokeratosis palmaris et plantaris disseminata; DSAP, disseminated superficial actinic keratosis.
No funding was received.