Porokeratosis refers to a clinically heterogeneous group of disorders, until now considered as keratinization disorders (Table 1). Recently, heterozygous germline mutations have been reported, causing pathogenic variants in the mevalonate pathway,1,2 which is crucial for cholesterol synthesis—an essential component for the formation of extracellular lipid lamellae in the stratum corneum. There has also been speculation about the possible role of germline mutations in the solute carrier 17 (SLC17) family in disseminated superficial actinic porokeratosis (DSAP). However, these germline mutations do not seem to be sufficient to produce significant structural or functional abnormalities in the epidermis. A “second hit” is necessary for the condition to manifest,3 with potential triggers including UV radiation, immunosuppressive therapies, certain systemic diseases, infections, or drugs.4,5
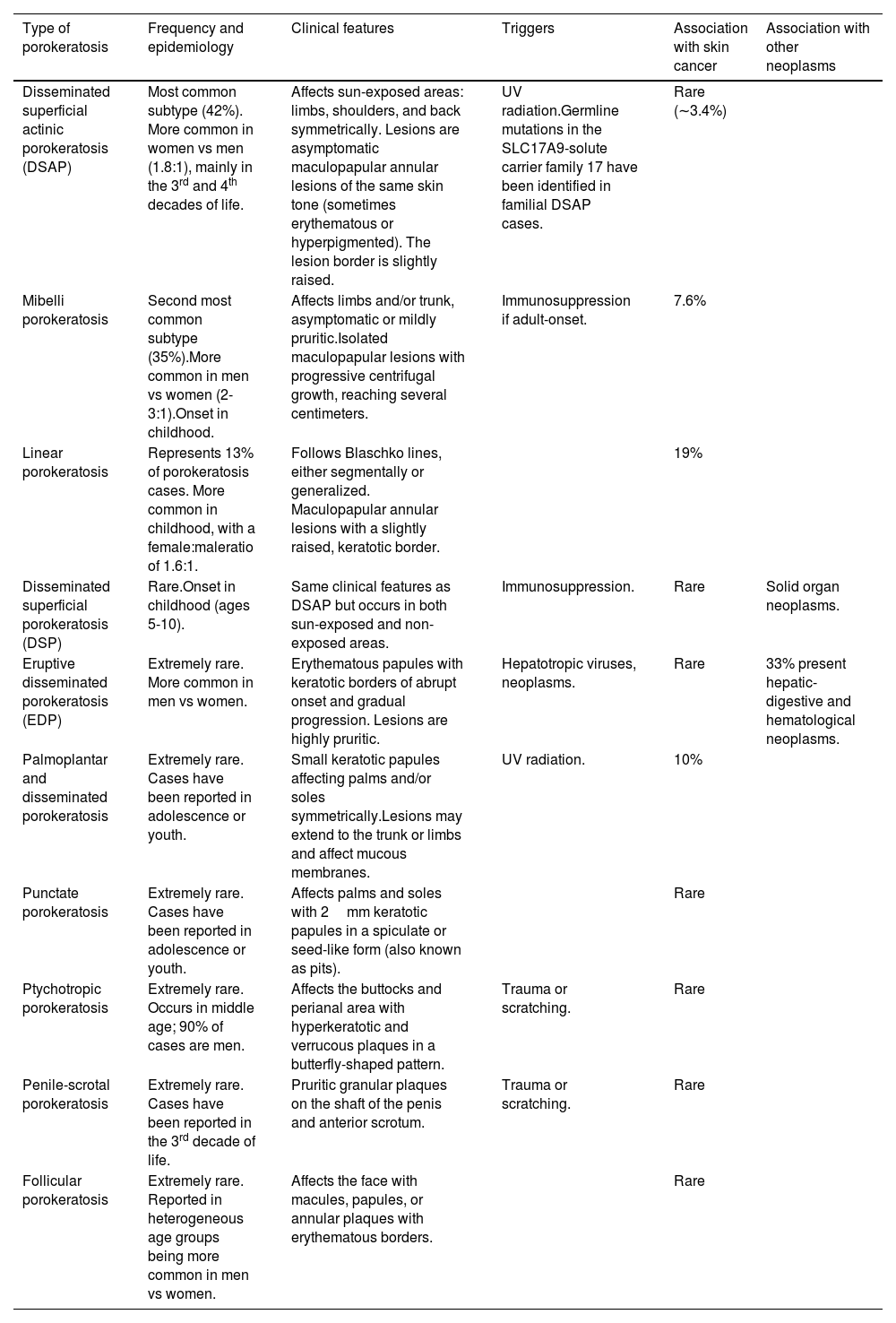
Types of porokeratosis and their main characteristics.
| Type of porokeratosis | Frequency and epidemiology | Clinical features | Triggers | Association with skin cancer | Association with other neoplasms |
|---|---|---|---|---|---|
| Disseminated superficial actinic porokeratosis (DSAP) | Most common subtype (42%). More common in women vs men (1.8:1), mainly in the 3rd and 4th decades of life. | Affects sun-exposed areas: limbs, shoulders, and back symmetrically. Lesions are asymptomatic maculopapular annular lesions of the same skin tone (sometimes erythematous or hyperpigmented). The lesion border is slightly raised. | UV radiation.Germline mutations in the SLC17A9-solute carrier family 17 have been identified in familial DSAP cases. | Rare (∼3.4%) | |
| Mibelli porokeratosis | Second most common subtype (35%).More common in men vs women (2-3:1).Onset in childhood. | Affects limbs and/or trunk, asymptomatic or mildly pruritic.Isolated maculopapular lesions with progressive centrifugal growth, reaching several centimeters. | Immunosuppression if adult-onset. | 7.6% | |
| Linear porokeratosis | Represents 13% of porokeratosis cases. More common in childhood, with a female:maleratio of 1.6:1. | Follows Blaschko lines, either segmentally or generalized. Maculopapular annular lesions with a slightly raised, keratotic border. | 19% | ||
| Disseminated superficial porokeratosis (DSP) | Rare.Onset in childhood (ages 5-10). | Same clinical features as DSAP but occurs in both sun-exposed and non-exposed areas. | Immunosuppression. | Rare | Solid organ neoplasms. |
| Eruptive disseminated porokeratosis (EDP) | Extremely rare. More common in men vs women. | Erythematous papules with keratotic borders of abrupt onset and gradual progression. Lesions are highly pruritic. | Hepatotropic viruses, neoplasms. | Rare | 33% present hepatic-digestive and hematological neoplasms. |
| Palmoplantar and disseminated porokeratosis | Extremely rare. Cases have been reported in adolescence or youth. | Small keratotic papules affecting palms and/or soles symmetrically.Lesions may extend to the trunk or limbs and affect mucous membranes. | UV radiation. | 10% | |
| Punctate porokeratosis | Extremely rare. Cases have been reported in adolescence or youth. | Affects palms and soles with 2mm keratotic papules in a spiculate or seed-like form (also known as pits). | Rare | ||
| Ptychotropic porokeratosis | Extremely rare. Occurs in middle age; 90% of cases are men. | Affects the buttocks and perianal area with hyperkeratotic and verrucous plaques in a butterfly-shaped pattern. | Trauma or scratching. | Rare | |
| Penile-scrotal porokeratosis | Extremely rare. Cases have been reported in the 3rd decade of life. | Pruritic granular plaques on the shaft of the penis and anterior scrotum. | Trauma or scratching. | Rare | |
| Follicular porokeratosis | Extremely rare. Reported in heterogeneous age groups being more common in men vs women. | Affects the face with macules, papules, or annular plaques with erythematous borders. | Rare |
Source: Adapted from Vargas-Mora et al. 4
A relationship between porokeratosis and skin cancer has been suggested,4 but to date, large epidemiological studies confirming this association have not been conducted.4
Inci et al.5 conducted a cohort study to investigate the association between porokeratosis and malignant skin neoplasms (squamous cell carcinoma [SCC], basal cell carcinoma [BCC], and melanoma [MM]). They selected a local cohort (n=108) of patients diagnosed with porokeratosis at Sahlgrenska University Hospital (University of Gothenburg, Sweden) from 2016 through 2020. The diagnosis of these patients, recorded in the Swedish National Health and Welfare Register, had a positive predictive value of 93.1%. They identified a total of 2277 patients diagnosed with porokeratosis between 2001 and 2020, with a median age of 68 years. About 71% of them were women. The incidence of porokeratosis observed in Sweden was 1.2/100,000 person-years; prevalence was 24.2/100,000 or ¼1.132 To assess whether there were any differences in the occurrence of skin tumors between cases and controls, researchers included all patients diagnosed from January 2006 through December 2015, including 10 randomly selected controls from the general population, matched by age, sex, and geographical location, followed until December 2019.
By cross-referencing data between cases and controls using the National Cancer Register, a significant increase in skin cancer was detected in the cases. Hazard ratio with a 95% confidence interval (CI) for patients with porokeratosis vs the control group was 4.3 (3.4-5.4) for SCC; 2.42 (2.0-3.0) for BCC; 2.8 (2.3-3.4) for patients exhibiting both SCC and BCC; and 1.8 (1.2-2.8) for MM.
Changes in the mevalonate pathway lead to cholesterol deficiency, which is necessary for producing, among other molecules, ubiquinone, steroid hormones, and prenylated proteins such as the RAS protein family. One hypothesis is that carcinogenesis in patients with porokeratosis occurs through the MAPK pathway, the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, or via p53, all involved in the carcinogenesis of MM and SCC. Although UV radiation, immunosuppressive therapies, or human papillomavirus may contribute as the “second hit” necessary to present the clinical signs of the disease, they may also contribute per se to carcinogenesis.5