Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the hair follicle characterized by nodules, abscesses, fistulas, and scars. Its main comorbidities include obesity, diabetes mellitus (DM), metabolic syndrome (MS), and polycystic ovary syndrome (PCOS).1
Metformin—the main treatment for type 2 diabetes (T2DM)—due to its ability to improve tissue sensitivity to insulin, also exhibits pleiotropic anti-inflammatory and immunoregulatory properties, according to the recent work by Petrasca et al.1 This study highlights the ability of metformin to attenuate glycolysis and the mammalian target of rapamycin (mTOR) pathway. Additionally, the ability of metformin to normalize the expression profiles of interleukin (IL)-17A, interferon-gamma, and IL-6 in neutrophils and skin explants from HS patients was demonstrated.2
The use of metformin as a second-line therapy in HS in clinical practice is a common thing.1 However, despite being included in the American (recommendation level 3) and British clinical practice guidelines (good practice point), no clinical trials have ever evaluated its efficacy profile.3,4
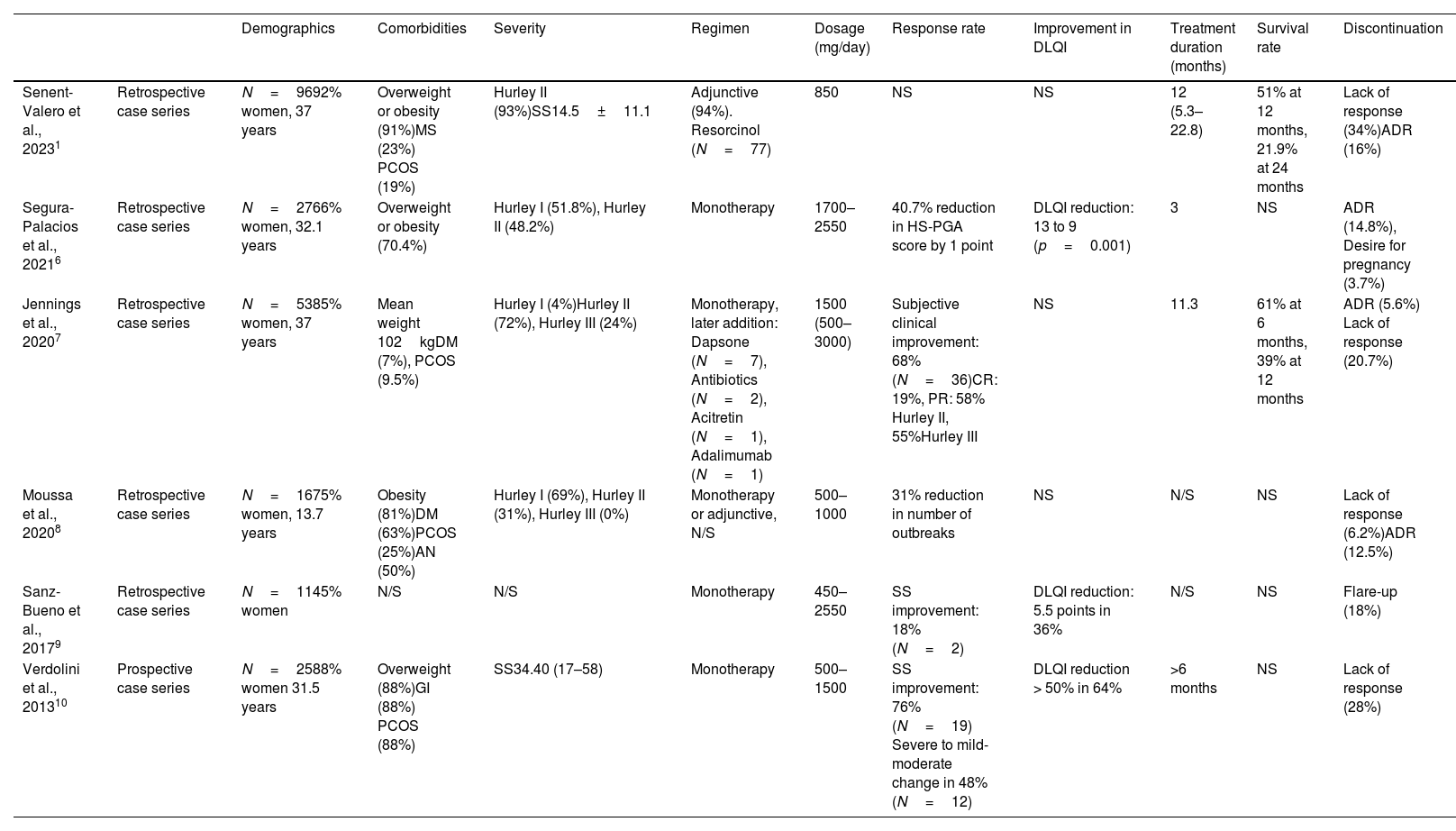
The current evidence comes from 6 case series (Table 1),1,5 covering a total of 228 patients treated with metformin, mostly women (83.3%), with a mean age of 38.4 years. More than 50% of the cases had overweight or obesity, and comorbidities such as PCOS (21.2%) or DM (9.6%). One series registered a total of 16 pediatric patients. The Hurley scale was the most widely used to define severity, with Hurley stage II being the most represented (139 patients).
Summary of published case series of patients with HS treated with metformin.
| Demographics | Comorbidities | Severity | Regimen | Dosage (mg/day) | Response rate | Improvement in DLQI | Treatment duration (months) | Survival rate | Discontinuation | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Senent-Valero et al., 20231 | Retrospective case series | N=9692% women, 37 years | Overweight or obesity (91%)MS (23%) PCOS (19%) | Hurley II (93%)SS14.5±11.1 | Adjunctive (94%). Resorcinol (N=77) | 850 | NS | NS | 12 (5.3–22.8) | 51% at 12 months, 21.9% at 24 months | Lack of response (34%)ADR (16%) |
| Segura-Palacios et al., 20216 | Retrospective case series | N=2766% women, 32.1 years | Overweight or obesity (70.4%) | Hurley I (51.8%), Hurley II (48.2%) | Monotherapy | 1700–2550 | 40.7% reduction in HS-PGA score by 1 point | DLQI reduction: 13 to 9 (p=0.001) | 3 | NS | ADR (14.8%), Desire for pregnancy (3.7%) |
| Jennings et al., 20207 | Retrospective case series | N=5385% women, 37 years | Mean weight 102kgDM (7%), PCOS (9.5%) | Hurley I (4%)Hurley II (72%), Hurley III (24%) | Monotherapy, later addition: Dapsone (N=7), Antibiotics (N=2), Acitretin (N=1), Adalimumab (N=1) | 1500 (500–3000) | Subjective clinical improvement: 68% (N=36)CR: 19%, PR: 58% Hurley II, 55%Hurley III | NS | 11.3 | 61% at 6 months, 39% at 12 months | ADR (5.6%) Lack of response (20.7%) |
| Moussa et al., 20208 | Retrospective case series | N=1675% women, 13.7 years | Obesity (81%)DM (63%)PCOS (25%)AN (50%) | Hurley I (69%), Hurley II (31%), Hurley III (0%) | Monotherapy or adjunctive, N/S | 500–1000 | 31% reduction in number of outbreaks | NS | N/S | NS | Lack of response (6.2%)ADR (12.5%) |
| Sanz-Bueno et al., 20179 | Retrospective case series | N=1145% women | N/S | N/S | Monotherapy | 450–2550 | SS improvement: 18% (N=2) | DLQI reduction: 5.5 points in 36% | N/S | NS | Flare-up (18%) |
| Verdolini et al., 201310 | Prospective case series | N=2588% women 31.5 years | Overweight (88%)GI (88%) PCOS (88%) | SS34.40 (17–58) | Monotherapy | 500–1500 | SS improvement: 76%(N=19) Severe to mild-moderate change in 48%(N=12) | DLQI reduction > 50% in 64% | >6 months | NS | Lack of response (28%) |
AN: acanthosis nigricans; DLQI: Dermatology Life Quality Index; DM: diabetes mellitus; HS-PGA: Hidradenitis Suppurativa Physician Global Assessment; GI: glucose intolerance; ADR: adverse drug reactions; MS: metabolic syndrome; PCOS: polycystic ovary syndrome; NS: not specified; N/S: non-significant.
There are significant disparities in the evaluation methods used, with standardized tools—Sartorius Score, Physician Global Assessment—being employed in only 3 of the studies. The others used activity descriptors (number of outbreaks, suppuration, and pain). Despite the reported heterogeneity, most studies reported clinical improvements in 31–76% of the patients. Lack of response was the most common cause for discontinuation (6.2–34%). Metformin was well tolerated, with transient digestive discomfort being the most reported adverse effect. Although the pediatric series described 2 cases of mood swings, they did not require discontinuation. Three studies described changes in the Dermatology Quality of Life Index (DLQI), and a reduction of 4 points turned out to be significant (p<0.01) in a series of 27 subjects. This same series showed a 12-month survival rate of 31%. Only 2 series have provided survival data, the most recent being the one by Senent-Valero et al., who showed drug persistence rates of 51% and 21.9% at 12 and 24 months, respectively, in a cohort of 96 patients, the largest published to this date.5
Therefore, despite the heterogeneity of the available data, metformin happens to be an affordable and well-tolerated therapeutic option with promising properties for patients with mild-to-moderate HS. The low percentage of patients with comorbidities (DM, PCOS) included in the series may underestimate the real effect of the drug in these subgroups. Although the collected data support the use of metformin in HS, further studies are needed to accurately evaluate safety and efficacy profile.
Conflict of interestThe authors declare that they have no conflict of interest.