In vivo reflectance confocal microscopy (RCM) is a relatively novel non-invasive tool for microscopic evaluation of the skin used prevalently for diagnosis and management of skin tumour. Its axial resolution, its non-invasive and easy clinical application represents the goals for a large diffusion of this technique. During the last 15 years, RCM has been demonstrated to be able to increase the sensibility and sensitivity of dermoscopy in the diagnosis of skin tumours integrating in real time clinic, dermoscopic and microscopic information useful for the definition of malignancy.
Despite to date, no large comparative studies on inflammatory skin diseases has been published in the literature, several papers already showed that RCM has a potential for the evaluation of the descriptive features of the most common inflammatory skin diseases as psoriasis, lupus erythematosus, contact dermatitis and others.
The aim of the application of this technique in non-neoplastic skin diseases has been prevalently focused on the possibility of clinical diagnosis confirmation, as well as therapeutic management. Moreover, the use of RCM as driver for an optimised skin biopsy has been also followed in order to reduce the number of unsuccessful histopathological examination.
In this review article we describe the confocal features of the major groups of inflammatory skin disorders focusing on psoriasiform dermatitis, interface dermatitis and spongiotic dermatitis.
La microscopia confocal de reflectancia (MCR) in vivo supone una relativamente novedosa herramienta de evaluación cutánea microscópica no invasiva que se emplea sobre todo para el diagnóstico y tratamiento de tumores de piel. Su resolución axial y su aplicación clínica sencilla y no invasiva representan los objetivos de una gran difusión de esta técnica. Durante los últimos 15 años, la MCR ha demostrado aumentar la sensibilidad y especificidad de la dermatoscopia en el diagnóstico de los tumores cutáneos de manera que se integre de manera simultánea la información clínica, dermatoscópica y microscópica relevante para definir el tumor maligno.
Hasta ahora no se han publicado estudios comparativos de enfermedades inflamatorias de la piel, varios artículos han mostrado que la MCR cuenta con potencial para la evaluación de las características descriptivas de la mayoría de las enfermedades inflamatorias cutáneas, tales como psoriasis, lupus eritematoso y dermatitis de contacto entre otros.
El objetivo de la utilización de esta técnica en enfermedades cutáneas no tumorales se ha centrado fundamentalmente en la posibilidad de confirmar el diagnóstico clínico, así como en el manejo terapéutico. Asimismo, se ha servido de la MCR como motor para una biopsia cutánea opmitizada para reducir el número de exploraciones histopatológicas ineficaces.
En este artículo de revisión se describen las características confocales de los principales grupos de trastornos cutáneos inflamatorios, centrándose en la dermatitis psoriasiforme, dermatitis de interfase y dermatitis espongiótica.
In vivo reflectance confocal microscopy (RCM) is a relatively novel non-invasive tool for microscopic evaluation of the skin that is prevalently used for skin tumours diagnosis and clinical decision management1–3 with prevalent application on melanocytic lesions. RCM provides “virtual” skin biopsies offering detailed microscopic changes involving the different skin layers with “new” en-face points of view with cellular-level resolution close to conventional histopathology. Recent prospective studies demonstrated that the systematic application of RCM as a second-level examination in skin oncology improve diagnostic accuracy, disclosing its potential as an alternative non-invasive, real time diagnostic method for pre-histologic diagnosis and patient management. High sensitivity and specificity values of some confocal criteria for melanocytic and non-melanocytic lesions have been identified demonstrating as the widespread use of RCM could spare numerous unnecessary biopsies for histopathologic examination.4
If in dermato-oncology RCM has been proved to provide information related to the nature of the skin lesions and its malignant potential, in inflammatory skin diseases RCM has been prevalently tested for the clinical microscopic correlation providing in real time microscopic features for clinical diagnosis confirmation. In detail, RCM has been used for the evaluation of several inflammatory skin diseases such as psoriasis, lichen planus and contact dermatitis5–7 describing specific confocal features supporting the clinical diagnosis useful for patient management.8,9 Data on confocal microscopy of single inflammatory skin diseases focusing mainly on confocal-histology correlation, therapeutic follow-up and only rarely on differential diagnosis have been published to date in the literature.10,11 Large comparative studies demonstrating the effective diagnostic and differential diagnosis value of RCM in these fields are still needed.
Following, we report a detailed description of the RCM features useful for the differentiation between the 3 main inflammatory diseases groups: psoriasiform, spongiotic and interface dermatitis. RCM features useful for discrimination between sub-entities of the main groups are also reported.
The RCM techniqueCommercially available RCM Vivascope 1500® (Lucid Technologies, Henrietta, New York, USA) and Vivascope 3000® operates with a diode Class 3A Laser (European version), at a wavelength of 830nm, with power less than 35mW at tissue level. The system included a 30× 0.9 NA water-immersion objective lens. Real time RCM operates by detecting back-scattered photons from the illuminated living tissue. The contrast in confocal images is provided by refractive index differences between cellular structures, subcellular structures and their surroundings.12 Melanin and keratin are highly refractive and to a lesser extent white blood cells, chromatin, collagen, and elastin in the dermis. RCM provides a cellular resolution that is similar to those of histopathology with lower magnification. The measured lateral resolution has been shown to be 0.5–1μm and the measured axial resolution less than 5μm. A drop of immersion oil or water has to be applied to the skin lesion in order to have a refractive index sufficiently close to that of the stratum corneum. The immersion lens requires to be used with water/water-gels placed between the window and objective lens with a refractive index close to that of the epidermis. The Vivascope 1500 needs to be attached to the skin with an adhesive ring to reduce artefacts during examination. Differently the Vivascope 3000, thanks to its ergonomic and the optic structure, does not need to be connected by the adhesive window to the skin, but can be moved free on the surface enabling to access “difficult” body sites with curved surfaces inaccessible to the Vivascope 1500. Details of this system have been reported in literature.13 Moreover, the intrinsic horizontal, in vivo RCM approach to the tissue, support the evaluation of the skin layer by layer from the top to the upper dermis similarly to as required in optical histology of scalp diseases. In specific, in order to obtain informative features about all the skin layers detecting the specific changes descriptive for the different inflammatory entities, the correct RCM acquisition method is generally made by four mosaics (usually 4mm×4mm is sufficient) taken respectively at the level of the stratum corneum, the spinosum, the dermo-epidermal junction (DEJ) and the upper dermis. One or more stacks (a vertical sectioning of the skin) moving from the stratum corneum to the deep limit of imaging (from 200 to 250μm according to the different anatomical sites and skin type of the patient) has been required in order to evaluate specific single microscopic criteria related to the thickness of the different skin layers. Moreover, during real-time imaging, video registrations can be acquired for analysis and record of dynamic processes such as blood flow in dermal vessels. Typically, leukocytes can be seen rolling through the vessel lumen.
Inflammatory diseasesOn the basis of the description of the histologic pattern, collection of RCM images showing microscopic feature involving the stratum corneum, epidermis and superficial dermis have been described. Later, using the pattern analysis method, RCM let the examiner to collect features on 3 main categories of inflammatory diseases: psoriasiform dermatitis, interface dermatitis, and spongiotic dermatitis.
Psoriasiform dermatitis refers to the inflammatory skin processes with an epidermal pattern characterized by thickened stratum corneum and epidermis associated with papillomatosis (elongation of the rete ridge). The prototypic entities of this group are plaque psoriasis (PP) and seborrheic dermatitis (SD).
Interface dermatitis includes those skin dermatoses in which an inflammatory process involves prevalently the DEJ, with injury and necrosis of basal cell keratinocytes associated with vacuolar or lichenoid changes. The most common interface dermatitis are represented by lichen planus (LP) and discoid lupus erythematosus (DLE).
Spongiotic dermatitis are characterized by the presence of inter- or intracellular oedema and prevalent infiltration of the epidermis due to inflammatory cells and perivascular infiltration in the upper dermis as in irritant and allergic contact dermatitis.
In the practise, in most of the cases, RCM let the reader to collocate the skin lesion in one of previous mentioned groups thanks to the identification of one of the major feature supporting or confirming the clinical suspect.
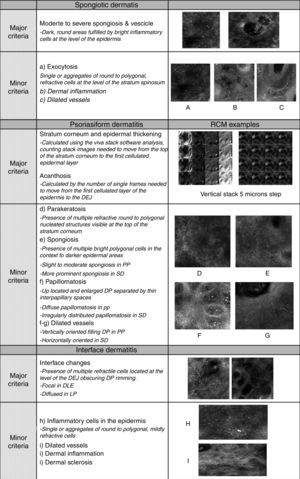
Later, in order to better define the differential diagnosis between inflammatory disease belonging to the same group, using RCM is also possible to collect additional criteria (named “secondary criteria”) involving the different skin layers for a better definition of a specific microscopic pattern (Fig. 1). The sensitivity and specificity of the method increased proportionally to the number of secondary confocal features composing the pattern of the clinically suspected skin disease.
In the following paragraph detail description of the main descriptors of the inflammatory groups and the secondary features useful for the differential diagnosis are described.
Spongiotic dermatitisThe main feature of spongiotic dermatitis on optical histology is the presence of inflammatory cells up migrated into the epidermis as single or in cluster forming intra-epidermal vesicles. This feature, when mild to moderate, can be also detected in the other major groups of inflammatory skin diseases. In this case the semi-quantitative estimation of the severity of the spongiosis (moderate to severe till the massive spongiosis) has to be more considered as more indicative for an acute spongiotic dermatitis. In the case of the detection of in-formation or already established vesicle involving the spinous layer, spongiotic dermatitis is substantially confirmed. Moreover, absence of interface changes as well as of sighs referring to papillomatosis help to confirm the clinical suspect of spongiotic dermatitis.
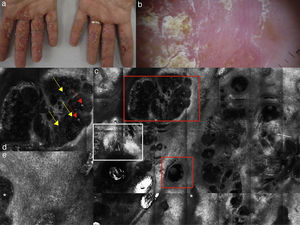
In detail, on RCM, spongiosis is visible as dark areas at the level of the epidermis in comparison with the surrounding epithelium, with broadband intercellular spaces and associated with round to oval bright cellular structures between keratinocytes spaces.14 When the spongiosis is massive, it presents as intra-epidermal vesicle seen on RCM as well demarcated dark spaces between granular and spinous keratinocytes, filled by “floating” inflammatory cells (Fig. 2). The last are visualized on RCM as round, small refractive cells between keratinocytes. Hyper-refractive necrotic keratinocytes can be also visualized inside the vesicles as mildly uniformly bright cellular structures floating in the dark area of the vesicle.
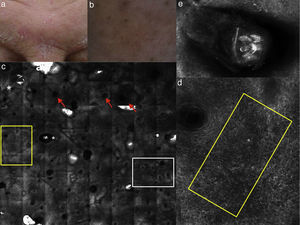
(a) Clinical picture of dyshidrotic eczema; (b) dermoscopic image disclosing erythema and scales; (c) RCM Vivablock mosaic taken at the level of the epidermis showing hyperkeratosis (white square) and clusters of intra-epidermal vesicles visible as well demarcated dark area (red squares); dark areas with the surrounding epithelium corresponding to spongiosis (asterisk); (d) RCM detail of intra-epidermal vesicle disclosing a vesicle with bright septa and bright cellular elements floating in the dark area of the vesicle (yellow arrows) and necrotic keratinocytes inside vesicle (red arrows); (e) RCM close-up of the epidermis showing an intra-epidermal vesicle (asterisk) that push the surrounding epidermis with loss of normal honey-combed pattern.
In spongiotic dermatitis, RCM can reveals disrupted corneum layer and detached corneocytes. Individual corneocytes may appear as detached highly refractile polygonal cells that correspond to subtle desquamation, reflecting the loss cohesiveness of corneocytes in response to contact irritants agents. Differentiation between irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) can be done through the evaluation of the changes involving the stratum corneum during time. In detail ICD reaction shows a pronounced superficial disruption of the stratum corneum after the exposure to contact irritants (less than 24h); this dynamic evolution of the stratum corneum involvement is generally absent in ACD.15 Dilated vessels and dermal inflammation can be visualized as secondary confocal features in both the subtype of spongiotic dermatitis. It has been shown that ICD and ACD differ in term of kinetic evolution, ICD reactions have a more rapid onset and shows a faster recovery compared to ACD.16,17 In addition it was demonstrated that RCM allows in the detection of subclinical reaction to in lab application of antigens when clinical features are absent or subtle, thereby verifying clinical readings of patch test (data non-published).
Psoriasiform dermatitisThe main psoriasiform inflammatory skin disorders in terms of frequency are known to be PP and SD, for this reason, in the literature, most of the papers are focused on the characterization of the confocal features of those.5,10,11
PP is a chronic inflammatory skin disease characterized on histopatology by acanthosis, hyperkeratosis, papillomatosis, an increased vascularization with lymphocytic/polymorphonuclated inflammatory response. RCM provides imaging of the whole stratum corneum and epidermis with the possibility of upper dermis evaluation trough the DP window. For that reason, RCM let the evaluation of all the skin compartments involved by the pathological process with valuable histopathology correlates, good reproducibility between observer and high grade of correspondence.18 Moreover, thanks to the possibility of detailed microscopic changes follow-up, recent studies on PP and other inflammatory skin diseases confirmed the usefulness of RCM for in vivo evaluation of therapeutic follow up and diseases progression.19–21
Describing more in detail the RCM descriptors of psoriasiform dermatitis, the main feature detected is the presence of thickened epidermis (>60–80μm) that have to be considered according to the different skin sites involved by the lesion and the skin type of the patient. At the superficial level (from 0 to 40μm) thickening of the stratum corneum is characterized by the co-existence of high refractive round to polygonal structures corresponding to parakeratosis typical for PP at histology. Technically, on RCM, thickening of stratum corneum and epidermis are evaluated using the Viva Stack software analysis. In particular, the number of single frames needed to move from the first cellulated layer of the epidermis to the DEJ can calculate epidermal acanthosis. Differently, hyperkeratosis can be measured counting stack images needed to move from the top of the stratum corneum and progressing deeper in 3–5μm step to the first cellulated epidermal layer.
Moreover, looking for secondary RCM microscopic features, up located, enlarged dermal papillae with thin interpapillary epidermal spaces, as signs of elongation of the rete-ridges (papillomatosis) are commonly detected and generally are indicative for PP (Fig. 3) instead of SD in which papillomatosis is more irregular. Different amount of spongiosis can be seen supporting the differential diagnosis between psoriasiform dermatitis.11 If PP is only mildly spongiotic, SD is generally characterized by moderate epidermal and dermal inflammation that has good correspondence with the more erythematous clinical presentation of the lesions (Fig. 4).
(a, b) Clinical pictures of plaque psoriasis involving lower limbs; (c) typical dermoscopic pattern of psoriasis characterized by red dots and glomerular vessels; (d) RCM Vivablock mosaic taken at the level of the dermo-epidermal junction showing hyperkeratosis (white square) and prominent papillomatosis, diffusely distributed; (e) RCM detail of non-rimmed, enlarged (white line) dermal papillae, increased in number and density separated by thin epithelial septa, and full filled by dilated blood vessels (yellow arrows).
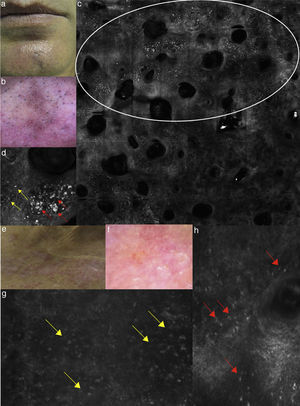
(a) Clinical image of SD involving the forehead; (b) dermoscopy showing erythema and scales with linear dilated blood vessels; (c) RCM Vivablock mosaic taken at the level of epidermis shows up migrated rimmed dermal papillae (white square), spongiosis (yellow square), sebaceous glands (red arrows) and adnexal structure with bright material inside corresponding to demodex folliculorum (asterisks); (d) RCM close up taken at the level of the epidermis reveals spongiosis associated with the presence of numerous inflammatory cells composed by small, reflactile, roundish cells corresponding probably to lymphocytes (yellow square) focally located around adnexal structure; (e) RCM detail of multiple roudish bright structures inside adnexal structure corresponding to posterior extremity of demodex folliculorum.
At the level of the upper dermis, prominent dark canalicular structures filling the DP are visible with vertical orientation in PP. Vascularization is detected to be differently distributed in SD showing a peculiar horizontal orientation of dilated blood vessels in the upper dermis usually located around adnexal structures. Detection of Demodex folliculorum inside sebaceous glands is constant in SD lesions, but absent in PP accordingly to the hyper-seborrhea characterizing SD and the high keratinocytes turnover of PP.
Interface dermatitisThe term interface dermatitis refers to skin dermatosis in which inflammatory process involves mainly the DEJ showing presence of focal or diffuse inflammatory cells infiltration.
DLE and LP are the prototypes of this group of inflammatory diseases as they are both characterized by the DEJ involvement; the first the interface involvement is seen as focal vacuolar changes at the basal membrane of the epidermis, the second as band-like infiltrate at the level of the upper dermis with erosion of the basal layer.
RCM have been tested for interface dermatitis defining its real power in detection of the major and minor descriptors characterizing this group of inflammatory skin diseases.6,22
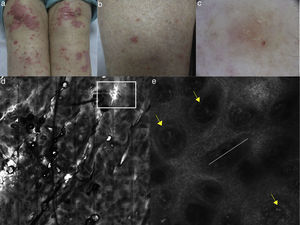
On RCM, the both histologic features are visualized as presence of multiple refractive cells obscuring the papillary rims, usually visible in normal conditions (Fig. 5). In detail, papillary rims are obscured by the presence of the inflammatory cells infiltrate almost completely involving the junction, with a total obliteration of the ring-like structures around DP that appeared non-edged and non-rimmed. According to the horizontal approach of RCM to the skin tissue, the detectable major difference in the DEJ involvement is showed by focal interface changes in DLE, and sheets of inflammatory cells distributed along the front of the lesion in LP as expression of the lichenoid infiltrate.
(a) Clinical picture of lichen planus; (b) dermoscopy reveals the presence of diffused blue-grey pigmentation arranged in a network around adnexal structures typical of a late stage lichen planus; (c) RCM Vivablock mosaic showing interface changes (white circle) with the presence of diffused inflammatory cells infiltrate, focused around adnexal structure, composed by numerous brightly reflactile, plump, oval to polygonal cells corresponding to melanophages (red arrows) and lymphocytes (yellow arrows); (e) clinical image of DLE; (f) dermoscopy shows teleangectasias associated with erythema; (g) RCM single frame at the level of the DEJ showing the obscuration of the DEJ by inflammatory cells (yellow arrows); (h) RCM detail of inflammatory process located around the adnexal structure with loss of the normal honeycombed structures of the epidermis; the inflammatory infiltrate is composed mainly by small, refractile, roundish cells corresponding probably to lymphocytes (red arrows).
Considering the secondary features, inflammatory cells can be also detected at the level of the epidermis as well as in the upper dermis around vessels in both DLE and LP. The difference between the two is the prevalence of melanophages in LP (especially in late stage lesions) visible on RCM as polygonal, plump bright cellular elements, usually without a visible nucleus, located in the upper dermis. Presence of inflammatory cells around/inside the adnexal epithelium and epidermal disarray can be seen on RCM in both LP and DLE, but in the last, dilated hyperkeratotic infundibula are more commonly seen. When the lesion involves the scalp, adnexal structures infiltration represents the main expression of the interface change and in lichen planopilaris it can be the prevalent location of the inflammatory cells infiltration.23 Detection of a more prominent thickening of the dermal fibres is more distinctive and characteristic for DLE.
In summary discrimination between DLE and LP derives from the identification and distribution of interface changes (focal in lupus and diffuse in lichen) and collection of secondary RCM criteria allowing the possibility to discriminate these interface dermatitis from other groups of inflammatory skin disorders.
ConclusionAt this point, it is universally accepted that RCM can be applied practically to the clinical management of skin lesions thanks to its non-invasiveness, its high correspondence with optical histology and to the possibility to have an immediate clinic-confocal correlation.
Throughout the visualization of larger fields of epidermal tissue using the mosaics ranging from 2 to 8mm in diameter, epidermis as well as dermo-epidermal junction and upper dermis can be scanned. RCM let the possibility to identify the presence of confocal microscopic features specific for different inflammatory skin disorders describing both main and secondary descriptors characterizing the single entities. Moreover, in the clinical practice, the success of RCM is strictly related to the possibility to reduce the number of skin biopsies and the possibility to repeat the exam in different areas of the same lesion during the same confocal section and during disease lifetime.
Several studies demonstrating the high potential of RCM in treatment follow up for a better and more specific patient and therapeutic management has been already reported in literature.9,19–21 Those experiences focused on the translational efficacy of this method in the different anatomical sites as, for example, has been reported for interface dermatitis involving the skin, scalp and oral mucosa.24–26
Referring to the last, preliminary studies on healthy, pathological oral mucosa indicated the possible value of RCM for the evaluation of sensible anatomical sites as oral cavity.27–29
If the practical application of RCM on inflammatory skin condition is spreading during the last years, limits characterizing this relatively new indication for RCM are becoming more and more evident. In detail, a major limitation of RCM for inflammatory diseases is represented by the depth of imaging, confined to the upper dermis (250μm) without the possibility to visualize reticular dermis and consequentially the deepness of skin inflammatory diseases. Moreover, in severely acanthotic disorders the DEJ and upper dermis cannot be visualized limiting the microscopic evaluation to the epidermis. Another limit is represented by the impossibility to discriminate different leucocyte's sub-types limiting the interpretation of the inflammatory cell infiltrate.
Nevertheless the limits, the advantage of a real time, non-invasive microscopic examination of the skin provides provide useful information for an immediate clinical-microscopic correlation that can be used by the clinician at least for several clinical applications: major differential diagnosis, therapeutic follow-up, detection of the best skin site for the biopsy.
If the microscopic information provided by RCM are close to histology, thanks to its significant lateral resolution, application of this method to inflammatory skin diseases has to be considered not substitutive to histology, but as integration to clinical diagnosis.
Further applications and larger studies comprehensive of different skin inflammatory condition could extend the possibility of routine application of RCM in clinical-microscopic differential diagnosis and could solve the actual limits of in vivo confocal microscopy.
Conflict of interestThe authors declare that they have no conflict of interest.