Moisturizing products are widely used in conditions affecting skin hydration. However, the lack of scientific evidence leads to discrepancies and great variability in the recommendations used by different health professionals. The aim of this consensus document is to generate recommendations based on the evidence and experience of dermatologists to unify and facilitate the use of moisturizing products in the routine clinical practice.
Materials and methodsA 49-statement questionnaire on moisturizing products was prepared and, then, arranged in 5 blocks: (1) concept, (2) characteristics, (3) frequency and quantity, (4) product use and areas of application, and (5) special populations. Twenty-two expert dermatologists in the management of patients with eczema answered to the survey using a 2-round Delphi methodology (adding an item on the 2nd round).
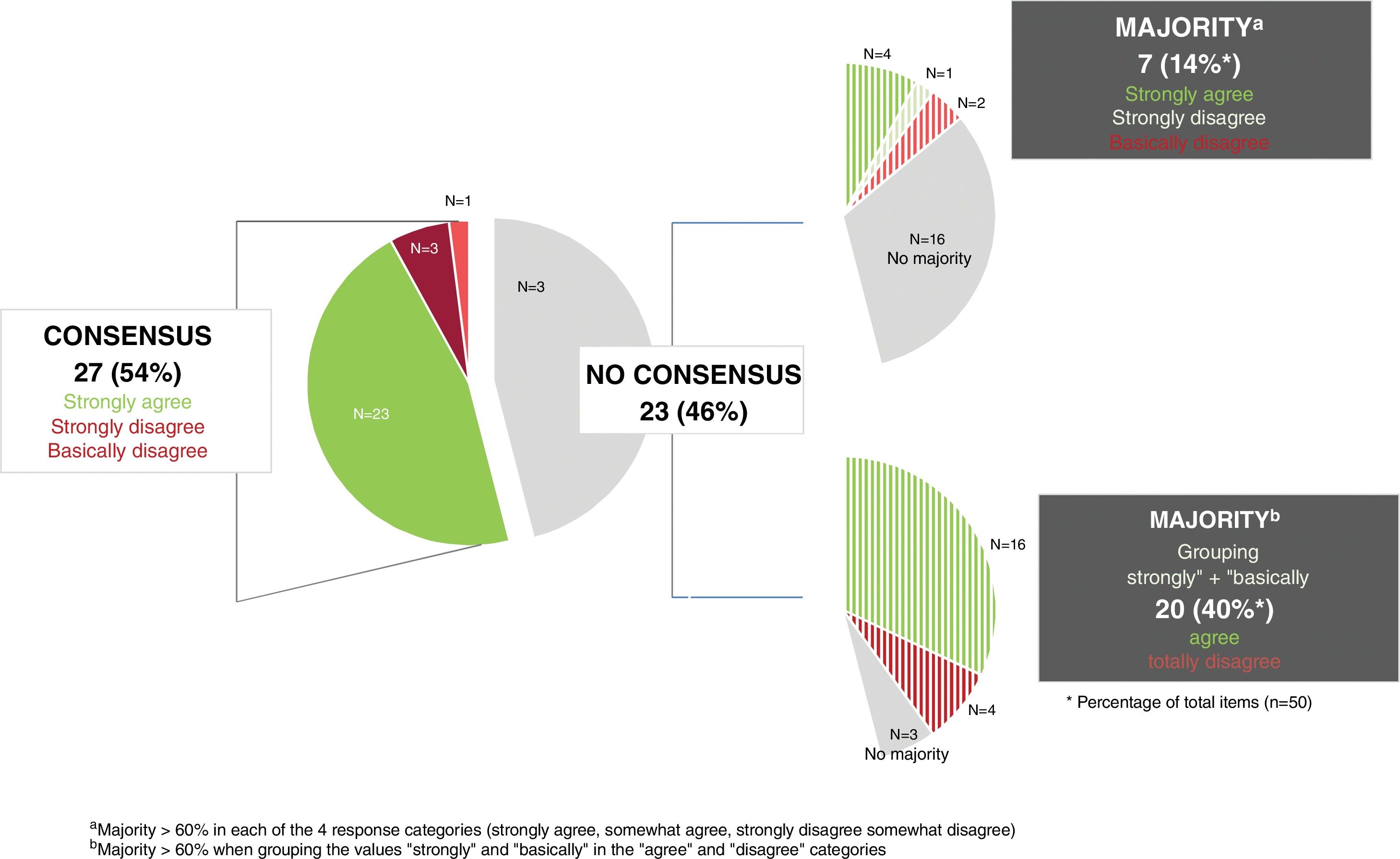
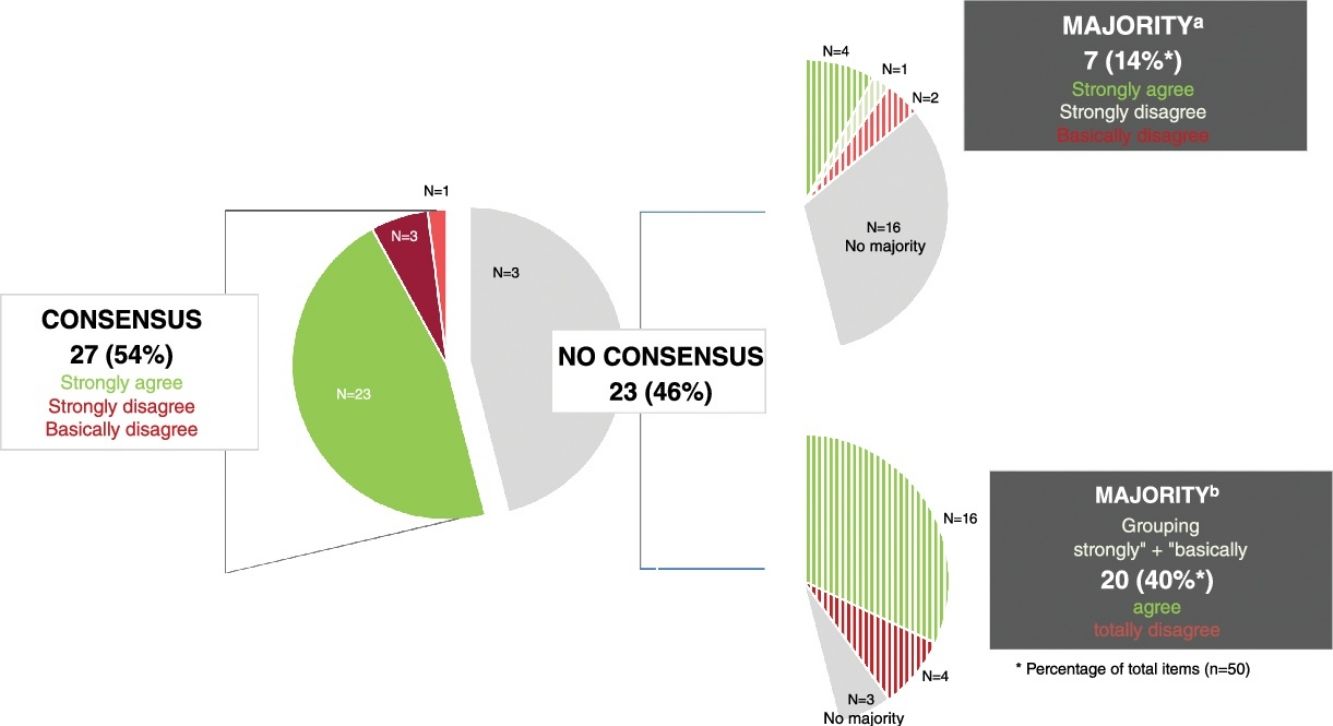
ResultsConsensus was reached on 27 statements (54%), most (n=23) via agreement. The highest level of agreement was reached in the blocks on quantity, product use and areas of application (77.8%), followed by the blocks on characteristics (73%) and frequency (62.5%). Regarding the blocks on concept and special populations, the level of consensus on the items proposed was 37.5% and 10%, respectively. Consensus on the use of emollients for xeroderma (71%) was higher vs atopic dermatitis (64%) and inflamed skin (33.3%).
ConclusionsConsensus recommendations can help all prescribers and improve the available evidence regarding their use.
Los productos hidrantes se utilizan ampliamente en enfermedades que afectan a la hidratación de la piel. Sin embargo, la falta de evidencia científica conlleva discrepancias y gran variabilidad en las recomendaciones de uso por los diferentes profesionales. El objetivo de este documento de consenso es generar recomendaciones basadas en la evidencia y experiencia de los dermatólogos para unificar y facilitar el uso de productos hidratantes en la práctica clínica.
Materiales y métodosSe elaboró un cuestionario con 49 afirmaciones sobre los productos hidratantes distribuidas en 5 bloques: 1) concepto; 2) características; 3) frecuencia, cantidad; 4) forma de uso y zonas de aplicación, y 5) poblaciones especiales. Fue respondido por 22 dermatólogos expertos en el manejo de pacientes con eccemas siguiendo la metodología Delphi modificada de 2 rondas (añadiéndose un ítem en la segunda ronda).
ResultadosSe consensuaron 27 (54%) ítems, la mayoría (n=23) en términos de acuerdo. El mayor porcentaje de consenso se alcanzó en el bloque sobre la cantidad, forma y zonas de uso (77,8%), seguido en los bloques de características (73%) y frecuencia (62,5%). En los bloques de concepto y de poblaciones especiales, el porcentaje de ítems consensuados fue del 37,5 y del 10%, respectivamente. El consenso sobre el uso de emolientes para xerodermia (71%) fue mayor que para dermatitis atópica (64%) y piel inflamada (33,3%).
ConclusionesLas recomendaciones consensuadas pueden ayudar a todos los prescriptores mientras se mejora la evidencia sobre su uso.
Proper skin hydration is essential to maintain its integrity and its role as a protective barrier. Therefore, topical moisturizing products are the first line of management for conditions affecting skin hydration, such as atopic dermatitis (AD), xerosis, and eczema.1,2 These products are generally classified into three groups based on their composition and mechanism of action3,4: (1) Humectants: hydrophilic substances that attract and retain water: (2) Emollients: lipophilic substances that fill the spaces between epidermal cells and prevent water evaporation; (3) Occlusives: lipophilic substances that create a waterproof layer on the skin surface, preventing evaporation.
Several clinical practice guidelines recommend the use of moisturizers5–7; however, there is significant variability and disagreement among health care professionals regarding which to use, when, where, and how.8 A unified criterion for recommending these products is lacking, often relying on variable factors such as cost, prescriber preference, patient preference, or formulation.8,9
The lack of agreement among dermatologists on moisturizers may be partly explained by the indiscriminate use of terms like emollient, humectant, and moisturizer, all associated with hydrating properties. These terms are often used interchangeably, even in scientific literature.10 Limited scientific evidence on these products, often of low quality,11 further contributes to the discrepancy. There is also insufficient evidence on the benefits of different formulations,5,12 which can differ in ingredients, hydration/lubrication capacity, antimicrobial, antipruritic, or anti-inflammatory properties, presentation, and cost.5,8,10,12–14
Same as it happens with other treatments, patients receiving differing recommendations for moisturizers may experience negative impacts. Changing recommendations can lead to mistrust in physicians and uncertainty about product applicability and usefulness.15 This uncertainty may promote inappropriate or inadequate use, reduce adherence, and diminish the benefits these products could provide. Furthermore, frequent product changes also impact the economic burden of treatment. Several studies in AD have reported 5-year costs per patient from €1100 up to €1575.16
This study aims to generate recommendations to guide and facilitate the prescription of moisturizers for specialists, minimizing the negative impacts that a lack of consensus has on patients.
MethodsTo reach a consensus, a 2-round modified Delphi method17 was employed, following RAND/UCLA recommendations.
Delphi methodA scientific committee including 2 dermatologists with over 30 years of experience prepared the questionnaire, addressing controversial aspects or uncertainties regarding moisturizers. A total of 5 topics were covered: (1) concept; (2) characteristics; (3) frequency; (4) quantity, method of use, and application areas; (5) special populations.
The degree of agreement for each item, presented as a statement, was assessed using 4 categories: strongly or somewhat disagree, and somewhat or strongly agree.
The questionnaire was distributed online to 22 dermatologists from the Spanish Research Working Group on Contact Dermatitis and Cutaneous Allergy (GEIDAC). The 1st round occurred from October to November 2022, and the 2nd one in December 2022.
Analysis and interpretation of resultsThe results of each item were presented as the percentage of responses in each of the 4 categories. Consensus was defined as ≥70%, and majority agreement as 60–70% (same criteria for both rounds).
After the first round, the scientific committee reviewed the responses. If a lack of consensus was attributed to unclear item wording, it could be reformulated and reintroduced as a new statement in round #2. The second-round questionnaire included reformulated items and those without consensus from round #1 (Table 1 and Fig. 1).
Items of the Delphi questionnaire on moisturizing products distributed to the expert panel (n=22).
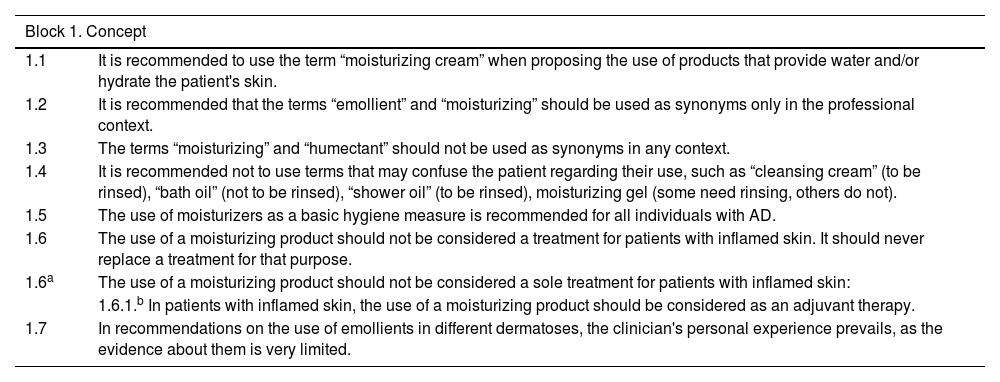
| Block 1. Concept | |
|---|---|
| 1.1 | It is recommended to use the term “moisturizing cream” when proposing the use of products that provide water and/or hydrate the patient's skin. |
| 1.2 | It is recommended that the terms “emollient” and “moisturizing” should be used as synonyms only in the professional context. |
| 1.3 | The terms “moisturizing” and “humectant” should not be used as synonyms in any context. |
| 1.4 | It is recommended not to use terms that may confuse the patient regarding their use, such as “cleansing cream” (to be rinsed), “bath oil” (not to be rinsed), “shower oil” (to be rinsed), moisturizing gel (some need rinsing, others do not). |
| 1.5 | The use of moisturizers as a basic hygiene measure is recommended for all individuals with AD. |
| 1.6 | The use of a moisturizing product should not be considered a treatment for patients with inflamed skin. It should never replace a treatment for that purpose. |
| 1.6a | The use of a moisturizing product should not be considered a sole treatment for patients with inflamed skin: |
| 1.6.1.b In patients with inflamed skin, the use of a moisturizing product should be considered as an adjuvant therapy. | |
| 1.7 | In recommendations on the use of emollients in different dermatoses, the clinician's personal experience prevails, as the evidence about them is very limited. |
| Block 2. Characteristics | |
|---|---|
| 2.1 | The choice of a moisturizing product for a patient with AD should be individualized, considering: |
| 2.1.a. Patient's age2.1.b. Body area2.1.c. Area to apply2.1.d. Ingredients2.1.e. Formulation2.1.f. Product price2.1.g. Patient preferences/experience2.1.h. Patient adherence2.1.i. Time of year | |
| 2.2 | For patients with AD, it is recommended to use: |
| 2.2.a. Moisturizing products that contain few ingredients2.2.b. Fragrance-free products | |
| 2.3 | For patients with AD, it is recommended to use moisturizing products that contain substances that protect or restore the skin barrier. |
| 2.4 | Avoid adding unnecessary or dubious ingredients to moisturizing products for patients with dermatitis, such as plant extracts, collagen, antioxidants, etc. |
| 2.5 | It is recommended to use leave-on moisturizers daily. |
| 2.6 | It is not recommended to use rinse-off moisturizers as the sole measure. |
| Block 3. Frequency | |
|---|---|
| 3.1 | It is recommended to use emollients: |
| 3.1.a. At least once a day in patients with active AD3.1.b. More than once a day in patients with active AD3.1.c. At least once a day in patients with AD, even without active lesions3.1.d. At least once a day in patients with xeroderma and itching3.1.e. More than once a day in patients with xeroderma and itching3.1.f. After a shower in patients with xeroderma3.1.g. Every time the patient feels that the skin is dry in patients with xeroderma3.1.h. At least once a day in any person, even without skin diseases | |
| Block 4. Quantity, usage method, and application areas | |
|---|---|
| 4.1 | It is recommended to use a sufficient amount of emollient to cover the entire body with a thin layer. |
| 4.2 | In patients with AD, it is recommended to use emollients on both the areas with lesions and the apparently healthy areas. |
| 4.3 | It is recommended to use emollients immediately after bathing or showering. |
| 4.4 | It is recommended to use emollients with completely dry skin, after drying with a towel. |
| 4.5 | It is not necessary to apply emollients to visibly healthy skin areas in patients with: |
| 4.5.a. AD4.5.b. Xeroderma | |
| 4.6 | It is recommended to apply emollients only to the areas with itching in patients with: |
| 4.6.a. AD4.6.b. Xeroderma | |
| 4.7 | For AD lesions, it is NOT recommended to use emollients if the patient experiences itching or burning after application. |
| Block 5. Special populations | |
|---|---|
| 5.1 | It is recommended that the elderly apply emollients: |
| 5.1.a. Preventively5.1.b. Only if they have xeroderma and/or itching | |
| 5.2 | It is recommended to use emollients in people with stasis dermatitis of the lower limbs. |
| 5.3 | It is recommended to use emollients preventively in all healthy neonates to avoid developing AD. |
| 5.4 | It is recommended to use emollients preventively in neonates at high risk of developing AD. |
| 5.5 | It is recommended to use different emollients for children than for other age groups. |
| 5.6 | It is recommended to use emollients: |
| 5.6.a. To prevent diaper dermatitis5.6.b. As an adjuvant therapy for diaper dermatitis | |
| 5.7 | It is recommended to use emollients to prevent stretch marks during pregnancy. |
| 5.8 | It is recommended to use emollients to prevent complications in the areolas during breastfeeding. |
AD, atopic dermatitis.
Results were analyzed by an agency independent of the scientific committee and the expert panel. The scientific committee then prepared and approved the final manuscript.
ResultsThe initial questionnaire on moisturizers included 49 items, increasing up to 50 in round #2 after adding a new item under the “Concept” section (1.6.1) (Table 1).
Overall, 27 out of 50 items (54%) in the final questionnaire achieved consensus (Fig. 1). The section with the highest percentage of consensus items was Section 4: “Quantity, method of use, and application areas,” followed by Sections 2: “Characteristics,” 3: “Frequency,” 1: “Concept,” and 5: “Special populations,” which had the lowest consensus (1 of 10 items).
During round #1, 16 of the 49 items achieved consensus. In round #2, item 1.6 was reformulated, and a new item (1.6.1) was added to the “Concept” section. The original item 1.6 was excluded from the results analysis.
The only item achieving consensus in round #1 on the terms used for moisturizers (Supplementary data, Fig. 1S; Item 1.4) referred to avoiding terms that might confuse patients about their use. In round #2, most panelists recommended using the term “moisturizing cream”, rejecting the use of “humectant” as a synonym and restricting “emollient” to professional contexts only.
Most items on “Characteristics and General Use” of moisturizers achieved consensus (Supplementary data, Fig. 2S).
Consensus was reached on 14 out of 22 items (64%) related to moisturizers for AD patients. Six of the remaining 8 items achieved majority agreement by combining the “somewhat agree” and “strongly agree” categories. The 2 items without consensus or majority referred to usage frequency (3.1.b) and price as a determining factor for product choice (2.1) (Supplementary data, Fig. 3S).
Five (71%) out of 7 items related to moisturizers for xerosis achieved consensus. The remaining 2 achieved majority agreement by combining “somewhat agree” and “strongly agree” categories (Supplementary data, Fig. 4S).
For items related to moisturizers for different skin conditions and special populations (Supplementary data, Fig. 5S), most experts highlighted a lack of evidence in dermatoses. Consensus was reached for 1 of 2 items on inflamed skin, rejecting moisturizers as the sole treatment (reformulated item 1.6) and agreeing on their use for stasis dermatitis of the lower limbs (item 5.2). While most respondents agreed on all items regarding preventive use, none achieved consensus.
DiscussionThis study presents the first consensus on moisturizers among Spanish dermatologists.
As stated by most participants, the limited scientific evidence on moisturizers, both in clinical practice11 and in dermatological guidelines, puts the clinician's personal experience ahead of the recommendations for their use in various dermatoses. This lack of evidence was confirmed by the low percentage of consensus (55% of items), highlighting the discrepancies in the management of moisturizers among health care professionals, which impacts clinical decisions.
The consensual items allow for the establishment of a series of recommendations, discussed below (Supplementary data, Table S1).
ConceptsRecommendation 1: The information provided by health care professionals about the product and its use should be clear. It is appropriate to use “moisturizing cream” with patients, limiting the use of “emollient” and “hydrating” as synonyms among professionals,10,14 as reflected in the use of both terms in the questionnaire items.
The terms “hydrating” and “humectant” are not interchangeable, in accordance with their mechanisms of action: creating a protective layer/barrier to prevent evaporation or attracting water to the skin, respectively.3,4,10,14
Characteristics and application of moisturizersRecommendation 2: To improve the efficacy of new moisturizers, brands include non-pharmacological active ingredients (e.g., plant extracts), targeting various mechanisms involved in the pathogenesis of AD (e.g., inflammation and skin barrier disruption).18 However, clinical evidence is still lacking,10 as well as studies comparing products with different ingredients, to demonstrate the efficacy of each.18
Recommendations 3 and 4: There is insufficient evidence to determine whether the amount of product applied to the skin is smaller with those that are rinsed off vs those applied directly.19 However, both dermatologists and most AD guidelines recommend using leave-on moisturizers. The lack of recommendation for rinse-off products as the sole measure may be justified by the absence (or minimal) benefit observed when adding bath-time moisturizers to the standard treatment for children with AD (BATHE study).20
Recommendation 5: The water content in the skin significantly increases when applying doses of moisturizers >1mg/cm2/application, being appropriate to up titrate this depending on the dryness of the skin and the patient's preferences.21
Recommendation 6: Using moisturizers immediately after bathing/showering is justifiable due to the increase in transepidermal water loss at that time.21 There was no consensus on applying them to dry skin (item 4.4), although this does not imply that they must be used on damp skin.
People with inflamed skin/dermatitisRecommendation 7: Moisturizers are considered adjuvant therapy. Numerous guidelines recommend applying these products after an appropriate pharmacological treatment to reduce inflammation, to improve skin barrier dysfunction.6,22 Moisturizers may help reduce inflammation, decreasing corticosteroid use.19
People with atopic dermatitisRecommendations 8 and 9: Health care professionals prefer products with fewer ingredients, fragrance-free, and without known allergenic preservatives. Fragrances offer no benefit and may lead to contact dermatitis due to sensitization.23
Recommendation 10: Other factors that most experts would consider, though no consensus was reached: (A) Price. Despite the high cost for patients, moisturizers1,16 have proven to be a cost-effective strategy in managing AD16; (B) Age. Specific products for those under 16 are not recommended; (C) Patient preferences/experiences; and (D) Time of year. Patient preference may be influenced by external climate and time of day.24
Recommendations 11–13: Guidelines state that moisturizers should be applied regularly, without specifying frequency, and in some cases, as needed by the patient.25 A recent study demonstrated that in patients with AD and xeroderma, skin water content was significantly higher when moisturizers were applied twice a day (after showering, in the morning and at night).21 This suggests that for patients with dry skin, the optimal application regimen should be, at least, twice a day.21 However, no consensus was reached on the need for more than 1 application per day in patients with active AD, which may be justified by the potential negative impact on patient adherence.
Consensus on the use of moisturizers in skin without active lesions, not visibly dry, is supported by the recommendation for proactive AD treatment that involves their use across the entire body (affected and unaffected skin) after lesions have been successfully treated with anti-inflammatory therapy.26 Additionally, using them without active lesions may be justified by preventing relapses.16
European clinical practice guidelines indicate that the direct use of moisturizers on inflamed skin is often poorly tolerated, so it is advisable to treat the flare first,26 which would justify not recommending their use on inflamed areas.
Patients with xerodermaRecommendations 14 and 15: Similar to AD, although it has been demonstrated that in patients with dry skin, moisturizers should be applied, at least, twice a day,21 experts did not reach consensus on whether they should be applied more than once a day in patients with xeroderma. Also, although there was no consensus, there was disagreement about restricting their application to itchy areas.
Patients with stasis dermatitis of the lower limbsRecommendation 16: These products are already included in the skincare routine for patients with stasis dermatitis.27
Two of the 3 items where consensus or majority was not reached (3.1 b and 3.1 h) referred to the frequency of emollient use in individuals with healthy skin or active AD. More evidence is needed in these situations to achieve dermatologists’ positioning. On the other hand, the failure to reach consensus or majority on item 5.6 (use of emollients as adjuvant therapy for diaper dermatitis), beyond the lack of evidence, may be influenced by the absence of pediatric dermatologists among the expert participants.
Preventive use of moisturizersThe reduction in consultation rates, hospitalizations, loss of productivity, and other medical costs described as a result of the preventive use of moisturizers16 positions it as a cost-effective strategy. It also reduces and delays flare-ups and the use of other topical treatments (corticosteroids), in addition to enhancing their efficacy when combined with them.28
Although there was no consensus, most experts would recommend preventive use in the elderly and children at high risk of AD. A recent meta-analysis in children concluded that they do represent a preventive strategy for AD, pending confirmation from larger studies.29 Preventive use in children without AD risk has not been determined, justifying the majority (but not unanimous) agreement not to use them as a preventive method for AD in all children.
In the elderly, despite the lack of evidence on the role of moisturizers in maintaining skin integrity,30 they would recommend preventive use, which is consistent with the negative opinion of using them only when xeroderma and pruritus are present.
Other preventive uses that most experts would recommend include the appearance of stretch marks during pregnancy (currently, topical products used to treat and prevent stretch marks contain emollients), complications in the areolas during lactation, and diaper dermatitis. Although moisturizers may create favorable conditions for optimal skin health under the diaper and promote faster recovery from diaper dermatitis, experts did not reach consensus on their use as adjuvant therapy for this condition.
This study confirms the lack of evidence on the use of moisturizers and the significant variability in their management in clinical practice. To help mitigate the negative impact that this lack of evidence may have on routine clinical practice, consensus-based recommendations are provided to aid in the unification of criteria for all health care professionals. Further studies are still needed to reduce discrepancies and improve evidence on the use of moisturizers.
FundingThis study was funded by Galderma.
The authors would like to thank the members of AEDV Spanish Research Working Group on Contact Dermatitis and Cutaneous Allergy (GEIDAC) who participated as panelists for their contribution to the document: Ana María Giménez-Arnau, Araceli Sánchez Grilo, Leopoldo Borrego Hernando, Susana Córdoba Guijarro, Enrique Gómez de la Fuente, Esther Serra Baldrich, Fátima Tous, Felipe Heras Mendaza, Francisco Javier Miquel Miquel, Francisco Navarro, Gemma Melé i Ninot, Inmaculada Ruiz, Javier Sánchez Pérez, José Carlos Armario Hita, José Manuel Carrascosa, Juan García Gavin, Maria Elena Gatica, Mercedes Rodríguez Serna, Pedro Mercader, Ricardo González Pérez, Tatiana Sanz Sanchez, and Violeta Zaragoza.
They also wish to thank Olga Isidoro and Got it for their support in the conception, development, and coordination of the project, and Lidesec for their collaboration while drafting this manuscript