The diagnosis of alopecia has traditionally relied on distinguishing between scarring and non-scarring forms, a differentiation that is not always straightforward from either a clinical or histopathological perspective. While the histopathological features of various types of alopecia are well established, recent reports have introduced new variants of non-scarring alopecia and identified histological features to achieve diagnosis. Some of these findings have therapeutic implications, while others provide novel pathogenic insights that enhance our understanding of alopecia. This article highlights recent advancements in the histopathology of non-scarring alopecia as reported in the literature.
El diagnóstico de la alopecia se ha basado tradicionalmente en la diferenciación entre formas cicatriciales y no cicatriciales, una distinción que no siempre es sencilla desde una perspectiva clínica o histopatológica. Aunque las características histopatológicas de los distintos tipos de alopecia están bien establecidas, publicaciones recientes han introducido nuevas variantes de alopecia no cicatricial y han identificado características histológicas que facilitan el diagnóstico. Algunos de estos hallazgos tienen implicaciones terapéuticas, mientras que otros proporcionan nuevas perspectivas patogénicas que mejoran nuestra comprensión de la alopecia. Este artículo destaca los avances recientes en la histopatología de la alopecia no cicatricial según lo informado en la literatura científica.
The classification of alopecia into scarring and non-scarring types is a fundamental diagnostic concept. Although this distinction may seem somewhat artificial, it has guided treatment strategies and provided more accurate prognostic information for patients. Scalp biopsy remains a valuable tool to diagnose alopecia, complementing clinical examination and trichoscopy.
However, distinguishing between scarring and non-scarring alopecia is not always straightforward, either clinically or histopathologically. In a recent study on chronic diffuse alopecia in women, biopsy results revealed scarring alopecia in 32.5% of cases, despite similar clinical presentations across the 32 patients studied.1
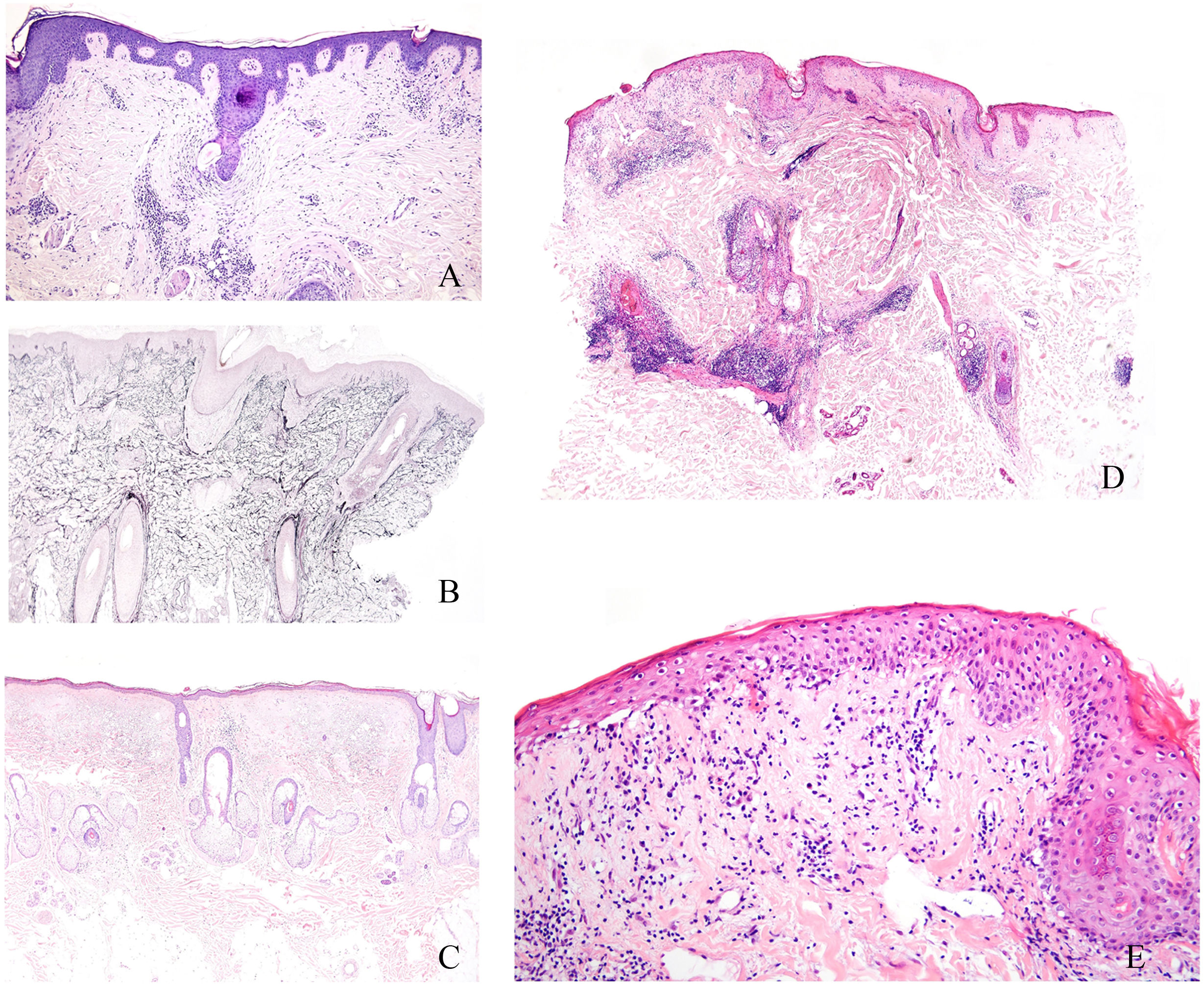
Histopathologically, scarring alopecia is primarily characterized by fibrosis, often perinfundibular, along with the destruction of elastic fibers, which may require special histochemical techniques for detection (Fig. 1A and B). Recently, Botega et al. described early histological markers that differentiate scarring from non-scarring alopecia before elastic fiber destruction occurs.2 Non-scarring alopecia typically exhibits a higher follicular density, with an increased number of non-terminal and miniaturized follicles2 (Fig. 1C). In contrast, scarring alopecia is often associated with dermal inflammatory infiltrates concentrated around the permanent portion of the follicle, frequently in a lichenoid pattern.2 Additional features include syringoma-like ductal proliferation, arrector pili muscle pseudohyperplasia, and reduced sebaceous gland density.2 Inflammatory infiltrates are particularly prominent in scarring alopecia associated with chronic discoid lupus erythematosus (CDLE). While CDLE is the primary scarring sign of alopecia in lupus, most alopecic presentations in lupus are non-scarring.
(A) Presence of concentric perinfundibular fibrosis, easily identifiable with haematoxylin and eosin staining, in a case of scarring alopecia. (B) Histochemical staining using the orcein technique, demonstrating the destruction of elastic fibres in the perinfundibular areas of a case of scarring alopecia. (C) Example of androgenetic alopecia (non-scarring), with marked follicular miniaturisation. (D) Biopsy of an alopecic patch in a patient with lupus erythematosus. Presence of a superficial and deep perivascular inflammatory infiltrate, along with perianexal involvement, dermoepidermal junction blurring, and epidermal atrophy. (E) Detail of the same case shown in figure LUPUS showing vacuolar degeneration of the basal layer of the epidermis with some apoptotic keratinocytes.
Historically, non-scarring alopecia in lupus erythematosus was considered a nonspecific or coincidental finding. However, recent studies have identified a specific form of lupus-associated non-scarring alopecia characterized by distinct histopathological features.3 Diffuse or patchy non-scarring alopecia and “lupus hair” may represent topographic variations of a single lupus-specific entity.3
Histopathological features of lupus-related non-scarring alopecia include epidermal atrophy, perivascular and periadnexal lymphoplasmacytic infiltrates (Fig. 1D), interface changes at the epidermal and/or follicular basal membrane (Fig. 1E), dermal mucin deposits, and immune deposits (IgG, IgM, IgA, C3, C1q) along the epidermal and/or follicular basal membrane.3
Clinically, lupus patients with non-scarring alopecia or lupus hair often exhibit a strong association with systemic lupus erythematosus (SLE) activity. These patients tend to respond well to SLE therapy and do not progress to scarring alopecia.3 In contrast, “linear and annular lupus panniculitis of the scalp” has a weaker association with SLE activity, and treatment responses may vary.
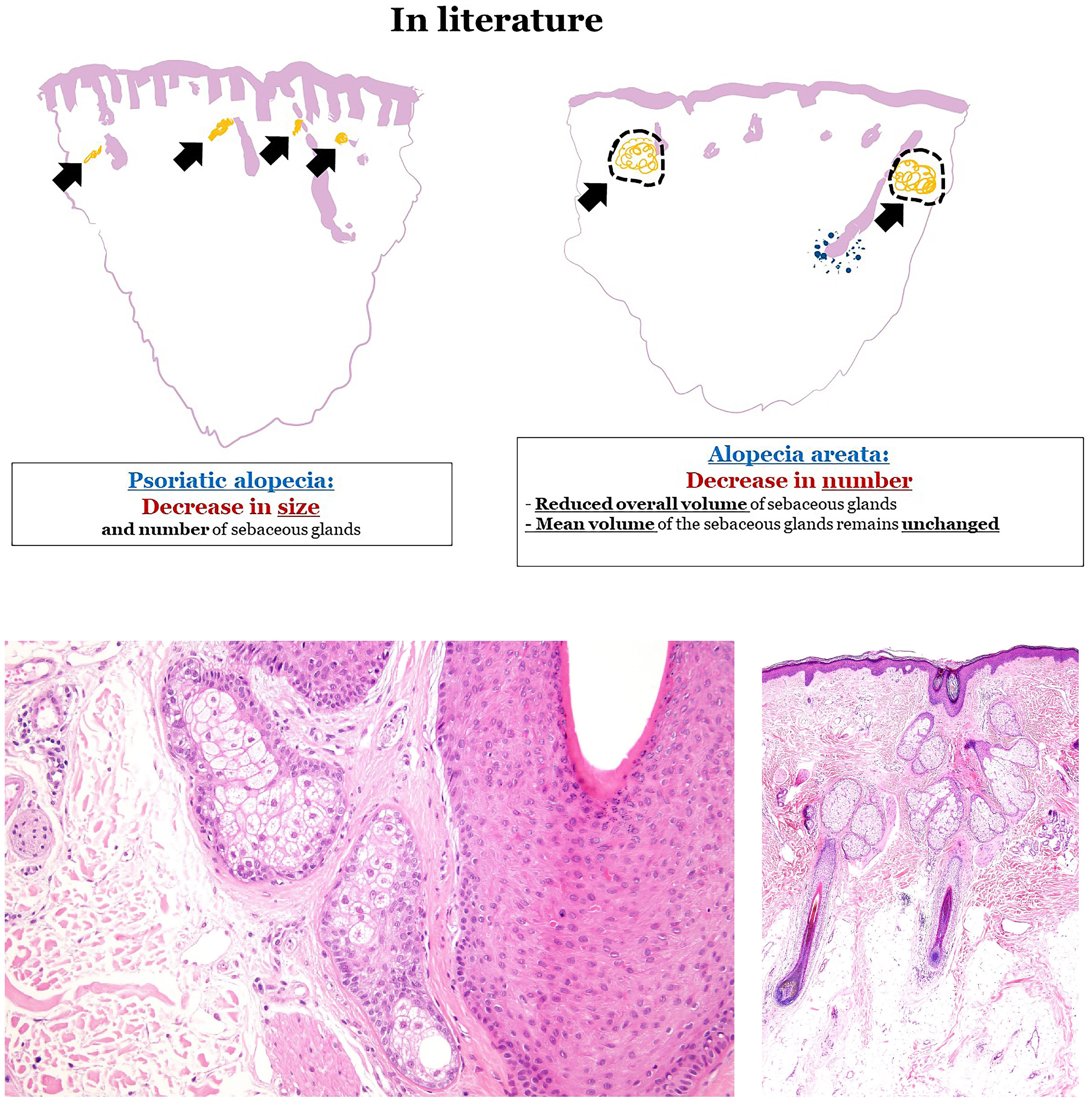
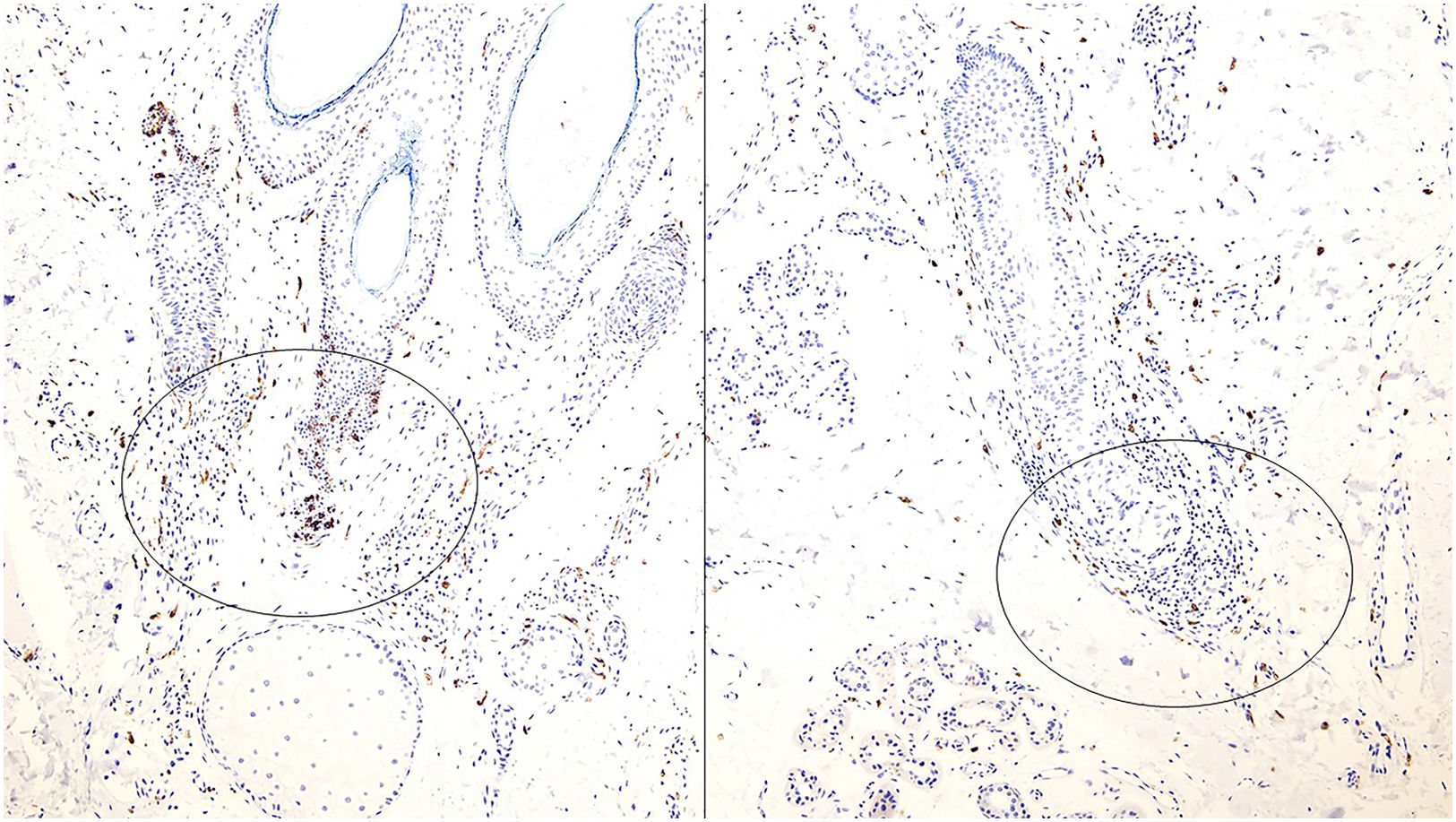
Emerging histological markers in non-scarring alopeciaThe sebaceous gland has emerged as a useful histological marker in distinguishing across different types of alopecia. For example, psoriatic alopecia typically exhibits a reduced size and number of sebaceous glands, whereas alopecia areata exhibits a reduced sebaceous gland count but retains normal gland volume (Fig. 2A). However, these changes are difficult to assess in small biopsies. A recent study found that sebaceous glands in psoriatic alopecia predominantly contain immature sebocytes (Fig. 2B), whereas those in alopecia areata consist mostly of mature sebocytes (Fig. 2C).4 While not pathognomonic, this finding may aid in diagnosis.
(A) Sebaceous glands in psoriatic alopecia and alopecia areata. In psoriatic alopecia, sebaceous glands show a reduction in size and number, while in alopecia areata, the mean volume of sebaceous glands remains unchanged with an overall reduced volume. (B) Sebaceous gland in an example of psoriatic alopecia, with an increased proportion of immature sebocytes, characterized by smaller, more basophilic cytoplasm and less vacuolation vs mature sebocytes. (C) Sebaceous glands in an example of alopecia areata. Glands are predominantly composed of mature sebocytes with pale, vacuolated cytoplasm.
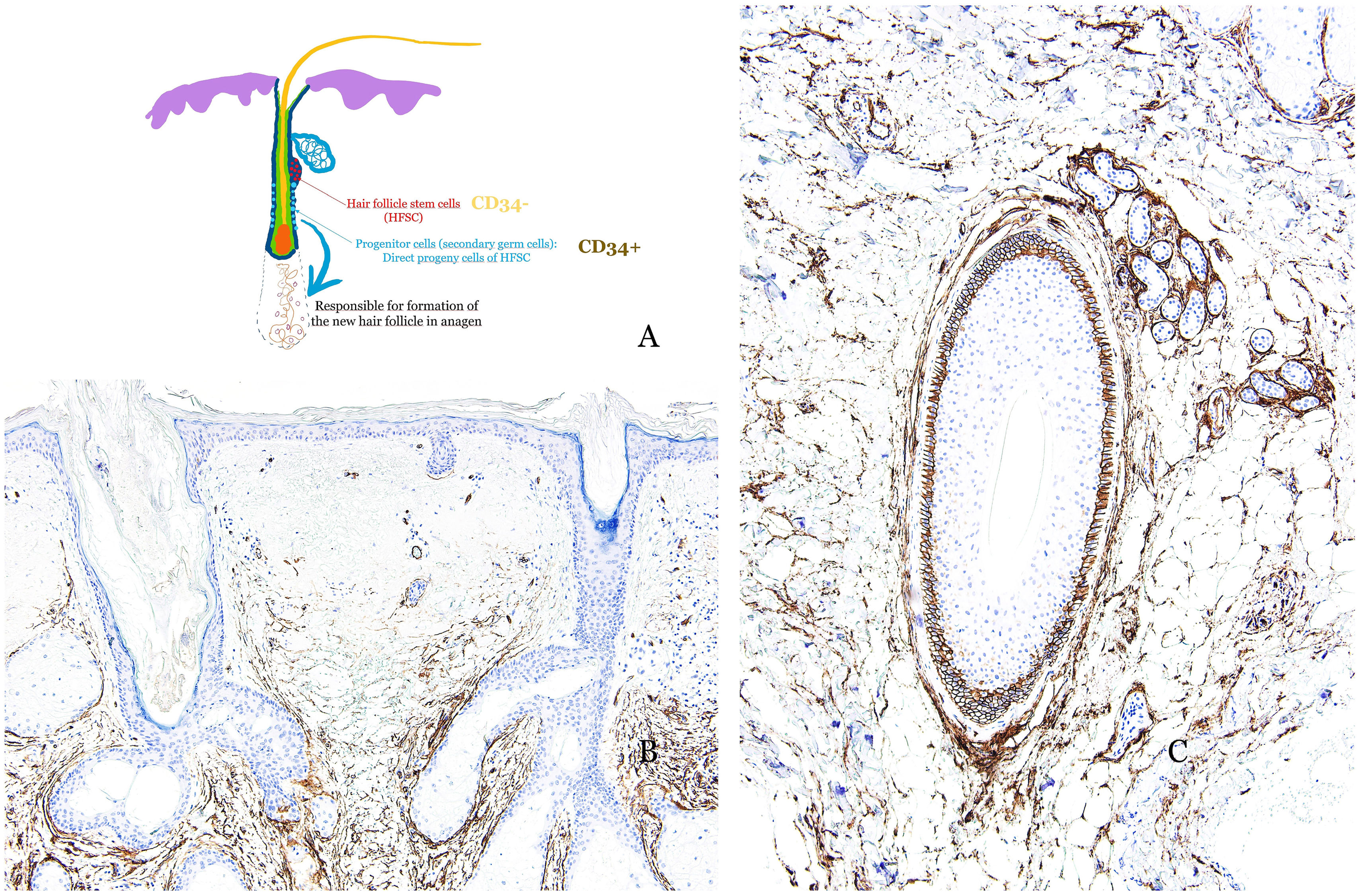
Another significant histological marker involves progenitor cells. Hair follicle stem cells, located in the bulge, generate CD34-positive progenitor cells in the outer root sheath, thus contributing to hair follicle regeneration during the anagen phase (Fig. 3A). In androgenetic alopecia, CD34-positive progenitor cells are absent (Fig. 3B), whereas they are abundant in alopecia areata (Fig. 3C).5–8
(A) Diagram illustrating the distribution of hair follicle stem cells (HFSC), primarily located in the bulge, as well as progenitor cells, considered as secondary germ cells and direct progeny of the HFSC (located along the outer root sheath). Progenitor cells are responsible for the formation of new hair follicles in the anagen phase. While hair follicle stem cells are CD34−, progenitor cells are CD34+. (B) Immunohistochemical staining for CD34 in a case of androgenetic alopecia, showing the absence of progenitor cells. (C) Immunohistochemical staining for CD34 in a case of alopecia areata, demonstrating strong expression in the outer root sheath of the hair follicle.
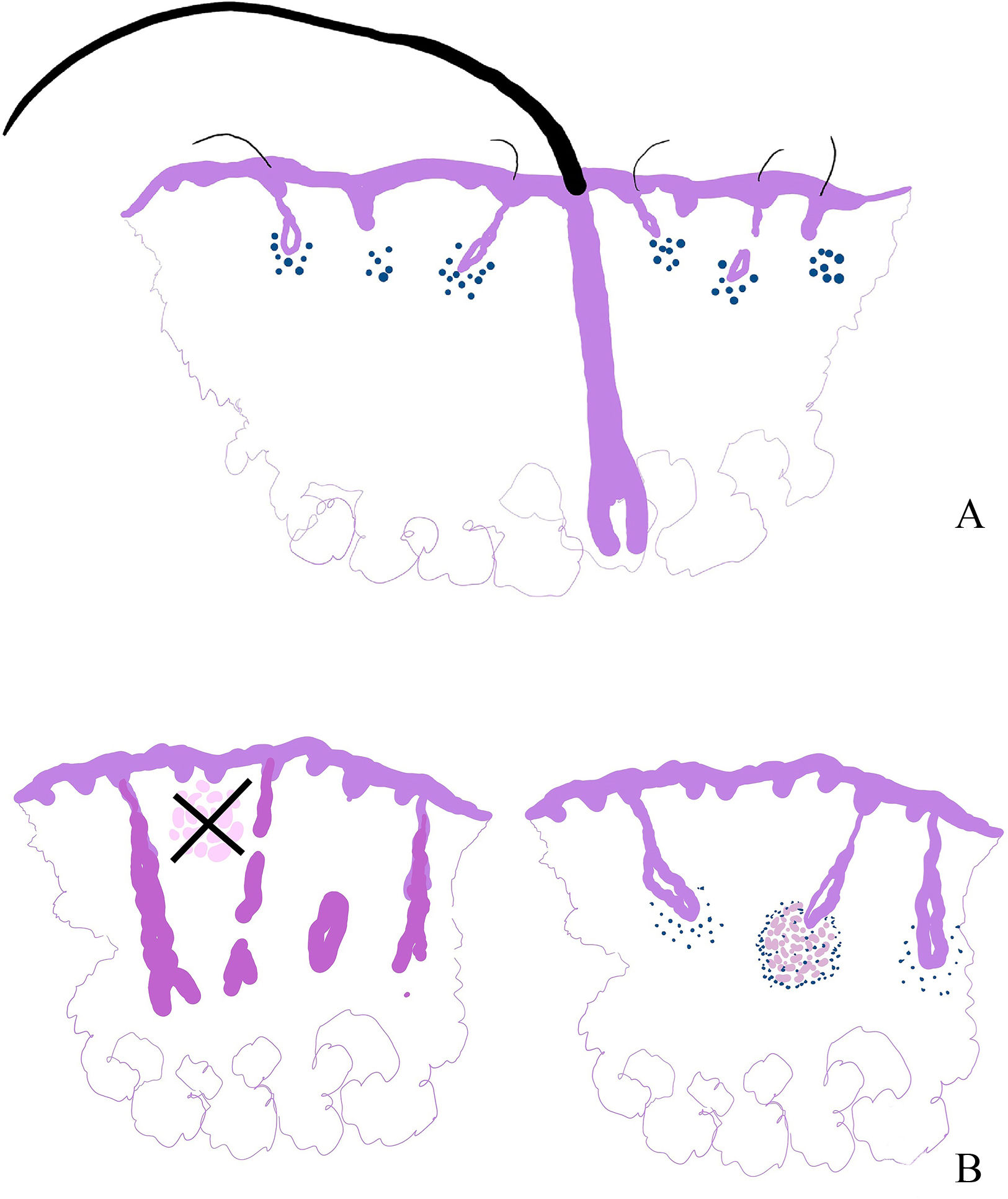
Refractory alopecia areata with single hairs has been recognized as a distinct subtype that clinically resembles frontal fibrosing alopecia.9 It presents with a receding hairline, single hairs with indistinct ostia, and empty follicles. The “single hairs” sign refers to preserved terminal hairs amidst a background of miniaturized follicles (Fig. 4A). This variant is typically resistant to conventional alopecia areata treatments, with limited response to corticosteroids, cyclosporine, and minoxidil.9,10
(A) Diagram showing an explanation of cases of alopecia areata of clinical presentation as refractory alopecia areata with single hairs. In these cases, a relatively preserved isolated terminal hair is seen amidst a population of miniaturized hairs affected by the disease. (B) The granulomatous variant of alopecia areata is not defined by the presence of granulomas in any location of the biopsy. Instead, granulomas are located in the hair follicle bulb.
Granulomatous alopecia areata, initially described in 2013, has recently been more firmly established.11 Unlike other alopecic conditions with dermal granulomas, this variant is defined by granulomas surrounding the hair bulbs, the hallmark site of inflammation in alopecia areata (Fig. 4B). Clinically, it presents with patchy alopecia, mild erythema, and a suddent transition from normal to damaged scalp areas.11,12,13
Other histopathological signs in some scarring alopecias have led to the description of apparently pathognomonic trichoscopic findings. This has been the case with folliculotropism of tumoral lymphocytes in mycosis fungoides, which has been associated with several interesting recently described trichoscopic signs, such as the presence of numerous pili torti, 8-shaped hairs, and hair shafts rapidly tapered over long sections.14
Histopathological findings with pathogenic and therapeutic implicationsRecent studies suggest that the pathogenic pathways in androgenetic alopecia differ between men and women.15 While male pattern hair loss primarily involves follicular miniaturization, female pattern alopecia is characterized by a reduced hair count per follicular unit. In advanced male alopecia, the pathogenic mechanism may shift from follicular miniaturization to decreased follicular density.
Mast cells have also been involved in the pathogenesis of alopecia areata16,17 (Fig. 5). Neural signals may stimulate mast cells, triggering CD8+ cytotoxic lymphocyte activity, which drives the inflammatory response in alopecia areata (Fig. 5). Targeting mast cells could offer new therapeutic avenues.
In pediatric alopecia areata, treatment is challenging due to the lack of approved therapies for children. A recent case report described successful treatment of a 9-year-old girl with alopecia areata and vitiligo on upadacitinib and narrow-band UVB therapy.18,19
Another therapeutic aspect to consider in non-scarring alopecias involves alopecic forms that can grow due to aesthetic procedures.20 Various forms of these are examples of non-scarring alopecias, and the pathogenic mechanisms involved are varied. For instance, an excessive injection of hyaluronic acid filler can cause pressure-induced alopecia.
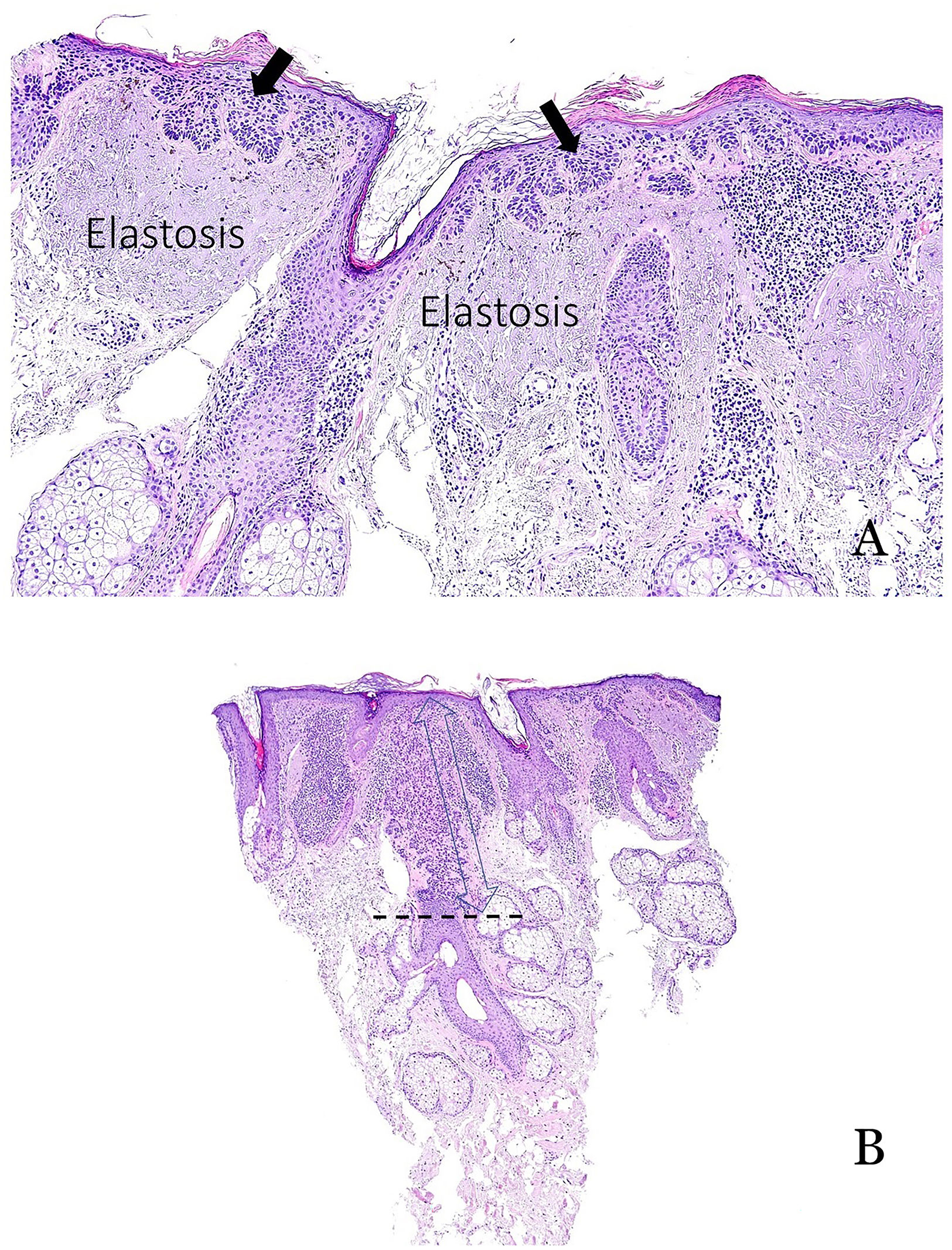
Late-stage alopecia and sun exposureIn advanced alopecia, chronic sun exposure can lead to actinic keratoses on the scalp, thus increasing the risk of squamous cell carcinoma.21 While actinic keratoses typically spare the pilosebaceous units, recent observations indicate that follicular involvement can occur (Fig. 6A and B). When present, the depth of follicular invasion should be documented, as extension to the lower third of the follicle is associated with a 100% risk of progression to invasive squamous cell carcinoma22 (Fig. 6B).
(A) Typical example of actinic keratosis affecting the epidermis but sparing the hair follicle. Prominent solar elastosis is observed in the dermis. (B) Example of actinic keratosis affecting the hair follicle. In such cases, it is important to include the depth of follicular involvement in the report.
Despite the long-established classification of alopecia into scarring and non-scarring types, recent histopathological discoveries are prompting revisions to traditional diagnostic criteria. New alopecia variants with distinct prognostic and therapeutic implications continue to emerge, highlighting the evolving nature of alopecia pathology and its clinical relevance.
Conflict of interestThe author declares no conflict of interest.