Moderate–severe atopic dermatitis (AD) has a significant impact on patients’ lives, with many requiring systemic treatment to manage symptoms (e.g., pruritus). Several drugs are used off-label to treat AD. This study describes sociodemographic/clinical characteristics, treatment patterns, health resource use (HRU) and costs in adults with AD who initiated systemic treatment or phototherapy in routine practice.
MethodsThis retrospective observational study of electronic medical records in the BIG-PAC database identified adults with prior diagnosis of AD (ICD-9: 691.8 or 692.9) starting oral corticosteroids, immunosuppressants, biologics or phototherapy between 01/01/2012 and 31/12/2016. Patients were followed for 3 years from treatment initiation, up to 31/12/2019. Data on patient characteristics, treatment patterns, HRU and costs were analyzed descriptively.
ResultsPatients (N=1995) had a mean age of 60 years, 64% were female, with a mean time of 23 years since diagnosis (84% were ≥18 years at AD onset). Main comorbidities were anxiety (38%), arterial hypertension (36%) and dyslipidemia (35%). Most patients used oral corticosteroids as first systemic (84%; median duration 29 days) and immunosuppressants in 13% of patients (median duration 117 days, 5% cyclosporine and 4% methotrexate). Half of patients required a second line systemic and 12% a third line. The use of immunosuppressants and biologics increased with treatment lines. About 13% of patients received systemic treatments continuously over the 3-year follow-up. The average 3-year per patient cost was 3835 euros, with an average annual cost of 1278 euros.
ConclusionsResults suggest a high comorbidity and economic burden in this real-world adult population with AD, and the need for systemic treatments indicated for use in AD.
La dermatitis atópica (DA) moderada-grave tiene un impacto significativo en la vida de los pacientes, muchos de los cuales requieren tratamiento sistémico para controlar los síntomas (p. ej., prurito). Algunos tratamientos son usados fuera de indicación. Este estudio describió características sociodemográficas y clínicas, patrones de tratamiento, uso de recursos sanitarios (URS) y costes asociados en adultos con DA que iniciaron tratamiento sistémico o fototerapia en la práctica habitual.
MétodosEste estudio observacional retrospectivo de historias clínicas electrónicas en la base de datos BIG-PAC identificó adultos con diagnóstico previo de DA (CIE-9: 691.8 o 692.9) que comenzaron con corticosteroides orales, inmunosupresores, biológicos o fototerapia entre el 01/01/2012 y el 31/12/2016. Se siguió a los pacientes durante 3 años desde el inicio del tratamiento, hasta 31/12/2019. Los datos sobre las características clínicas de los pacientes, patrones de tratamiento, URS y costes se analizaron de forma descriptiva.
ResultadosLos pacientes (N=1995) tenían una edad media de 60 años, el 64% eran mujeres, con una media de 23 años desde el diagnóstico (84% tenían≥18 años al inicio de la DA). Las principales comorbilidades fueron ansiedad (38%), hipertensión arterial (36%) y dislipidemia (35%). La mayoría de los pacientes utilizaron corticosteroides orales como primer tratamiento sistémico (84%; duración media 29 días) e inmunosupresores en el 13% de los pacientes (duración media 117 días, ciclosporina en el 5% y metotrexato en el 4%). La mitad de los pacientes requirieron una segunda línea de tratamiento sistémico y el 12% una tercera. El uso de inmunosupresores y biológicos aumentó simultáneamente con las líneas de tratamiento. Aproximadamente el 13% de los pacientes recibieron tratamientos sistémicos de forma continua durante los 3 años de seguimiento. El coste medio por paciente a 3 años fue de 3.835 euros, con un coste medio anual de 1.278 euros.
ConclusionesLos resultados sugieren una alta carga económica y de comorbilidad en esta población del mundo real de adultos con DA, y la necesidad de nuevos tratamientos sistémicos indicados para DA.
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disease1 that is characterized by intense itch and inflammatory eczematous lesions2. AD usually affects up to 10–20% of children, while the prevalence in adults is around 1–10% 3–5. A recent study revealed 7.2% prevalence of AD in the adult population in Spain 5. The moderate–severe forms may represent between 41 and 69% of patients with AD in Spain, based on different severity criteria 5–6.
Moderate–severe AD has an important economic impact, affecting quality of life and the psychosocial sphere of patients and their families, mainly due to the itching that can trigger sleep issues and psychiatric disorders (e.g. anxiety, depression)4–7. In adult patients, AD may also affect work productivity1,6.
Treatment aims to reduce symptoms and the number of recurrences while controlling the disease in the long-term8–10. At the time this study was conducted, for moderate–severe forms, treatments recommended in guidelines include topical corticosteroids and calcineurin inhibitors, phototherapy, short-term oral corticosteroids, conventional immunosuppressants (cyclosporine, mycophenolate mofetil, azathioprine and methotrexate, the last three unapproved for AD), and dupilumab monoclonal antibody 1,11,12.
Although several recent studies described disease burden in Spain 13,14, there is limited evidence on the use of treatments over time and the economic impact of the disease in routine practice conditions.
This study described sociodemographic and clinical characteristics, treatment patterns, health resource use (HRU) and costs in adults with AD who initiated treatment with systemic drugs or phototherapy in the real-world setting in Spain.
Patients and methodsStudy designA non-interventional, retrospective, longitudinal study based on electronic medical records in the Big-Pac database in Spain was conducted to describe sociodemographic/clinical characteristics, treatment patterns, HRU and associated costs. Adult patients with AD newly initiating treatment with a systemic drug (oral immunosuppressant, systemic corticosteroid, or biologic) or phototherapy between 01/01/2012 and 31/12/2016 (i.e., inclusion period; Fig. 1) were included. Patients were followed-up for 3 years from the date of first systemic treatment initiation or phototherapy (i.e., index date), up to 31/12/2019. A 2-year pre-index period was used to verify that no prior records of systemic drugs or phototherapy were documented.
Data confidentiality (anonymous and dissociated) was respected in compliance with Organic Law 3/2018, of December 5, on Protection of Personal Data and guarantee of rights (https://www.boe.es/eli/es/lo/2018/12/05/3). The study was approved by the Ethics Committee of the Consorci Sanitari de Terrassa (Barcelona, Spain) and conducted in accordance with the ethical principles of the Declaration of Helsinki and applicable laws and regulations in Spain.
Data sourceData were obtained from the Big-Pac database (Atrys Health-Real Life Data, Madrid, Spain), which collects computerized and anonymized patient medical records from primary and secondary care, records of drug dispensation and other complementary databases from seven regions in Spain. The population assigned to the health centers from which data were extracted included 1,867,108 inhabitants at the time of data extraction and may be considered representative of the Spanish population 15. The database is registered in The European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP®) (http://www.encepp.eu/encepp/viewResource.htm?id=29236#)
Study populationPatients meeting the following inclusion criteria at the index date were included: (a) had a record of AD diagnosis prior to the index date according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (691.8 – other atopic dermatitis and related conditions or 692.9 – contact dermatitis and other eczema, unspecified cause, with ‘allergic’ or ‘atopic’ in the ‘description’ field of diagnosis in the database); (b) started treatment with phototherapy or a systemic drug between 01/01/2012 and 31/12/2016; (c) aged at least 18 years, (d) patient included in the prescriptions program to obtain the medical prescriptions (with proven record of the daily dose, the time interval and the duration of each treatment being administered; 2 or more prescriptions during the monitoring period); (e) with 2 or more healthcare records; (f) continuously registered in the database for at least 3 years from the index date. Patients were excluded if they were (a) transferred to other centers, displaced or out of area, (b) permanently institutionalized, (c) had a terminal disease or (d) had a record of phototherapy or systemic medication in the 2-year pre-index period.
Only patients with an AD-related ICD-9-CM code documented for at least one prescription of systemic treatment during the inclusion period were included in the analysis.
Study endpoints and variablesSociodemographic and clinical data were obtained at or prior to the index date, including age, gender, BMI, time from AD diagnosis, age at AD onset. Most frequent (frequency ≥5%) clinically relevant comorbidities were described based on ICD-9-CM codes (Supplementary material Table 1). The mean (SD) number of comorbidities per patient was calculated. Topical AD treatments in the 6 months prior to the index date as well as selected concomitant treatments were extracted (Supplementary material Table 2; ATC codes16).
Systemic medications and phototherapy were obtained at the index date and throughout the follow-up period. Lines of treatment were built (Line 1, Line 2, Line 3 or Line>3). The first systemic treatment(s) or phototherapy initiated within the inclusion period (index treatment or first line therapy) prescribed in monotherapy or combination (when more than one drug was prescribed on the same day) were recorded. For subsequent treatment lines combination was defined as the concomitant use of two or more systemic drugs or phototherapy. Changes in the index or subsequent treatment lines include (a) discontinuation: end of systemic treatment or phototherapy with the occurrence of a gap in a series of successive dispensations≥60 days duration, (b) switching: end of systemic treatment or phototherapy with the start of a new systemic treatment within 60 days of the end of the prior treatment or (c) add-on: start of a new systemic treatment or phototherapy while the prior treatment is still being prescribed. Treatment duration for a specific drug was determined as the time (days) from initiation of that drug to the date of discontinuation or switching. Continuous systemic treatment during the 3-year follow-up was defined as the lack of discontinuation of systemic treatment (excluding oral corticosteroids) within this time period 17.
HRU was described based on resources recorded during the 3-year follow-up period, and used to calculate associated healthcare costs (medical visits, hospitalization days, emergency visits, diagnostic or therapeutic requests, drug prescription) and non-healthcare costs (i.e., days on sick leave). Only HRU associated with dermatological ICD-9-CM codes were considered for the analysis except for days of sick leave as they are not ICD-9-CM code linked. Costs were expressed as an average cost per patient (average/unit) per year, as well as the total cost in the 3 years follow-up period. Unit costs are detailed in Supplementary material Table 3 (year 2019). The rates were obtained from the analytical accounting, except for medication and days of sick leave. The medical prescriptions were quantified according to the public retail price per pack at the time of prescription 18. Days of sick leave were considered according to the average interprofessional salary 19. This study did not consider direct non-healthcare costs paid by the patient/family, as they are not recorded in the database.
Statistical analysesData were analyzed descriptively. For categorical variables, absolute and relative frequencies were provided. Continuous variables were reported using means, standard deviation (SD), median, and the 25th and 75th percentiles of the distribution (interquartile range). Results are presented overall and by index treatment. Where data were missing, the number of missing values was provided. The SPSSWIN program, version 23, was used for the analysis.
ResultsOf 1,867,108 patients in the Big-Pac database, 1995 were included in the analysis (Suppl. Fig. 1).
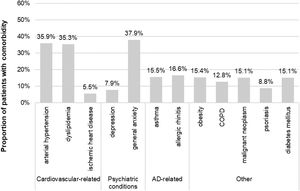
Sociodemographic and clinical characteristicsThe mean (SD) age at index was 59.8 (17.3) years and 64.4% of patients (n=1285) were female. Among those with available data (n=1189), the mean (SD) BMI was 29.5 (6.0) kg/m2. The mean (SD) time from AD diagnosis was 23.2 (13.4) years, 84% with adult-onset AD. The main comorbidities were general anxiety (37.9%), arterial hypertension (35.9%) and dyslipidemia (35.3%) (Fig. 2). Antibiotics, antihistamines and antidepressants were present in 46.8%, 29.9% and 26.7% of patients at the index date, respectively (Table 1).
Sociodemographic and clinical characteristics.
| Characteristics | N=1995 |
|---|---|
| Age (mean, SD), years | 59.8 (17.3) |
| Age groups, n (%) | |
| 18–44 years | 422 (21.2) |
| 45–64 years | 680 (34.1) |
| ≥65 years | 893 (44.8) |
| Gender, % women | 1285 (64.4) |
| Time from AD diagnosis (mean SD), years | 23.2 (13.4) |
| Age at AD onset, n (%) | 8.8 (1.3) |
| <14 years | 142 (7.1) |
| 14–17 years | 177 (8.9) |
| 18–60 years | 1445 (72.4) |
| >60 years | 231 (11.6) |
| BMI (mean, SD), kg/m2 | 29.5 (6.0)a |
| Number of chronic comorbidities per patient, mean (SD) | 2.5 (1.4) |
| Prior and concomitant treatments | |
| Non-systemic treatments in the 6 months prior to the index date,n (%) | |
| Topical corticosteroids | 629 (31.5%) |
| Topical immunomodulators | 38 (1.9%) |
| Concomitant treatments at the index date, n (%) | |
| Antihistamines | 596 (29.9%) |
| Antibiotics | 933 (46.8%) |
| Antidepressants | 528 (26.7%) |
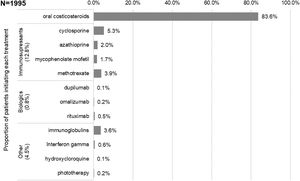
The first systemic treatments or phototherapy that patients initiated at the index date (i.e., Line 1) were (Fig. 3): oral corticosteroids (83.6%; n=1668), immunosuppressants (12.8%; n=256 mainly cyclosporine [5.3%] and methotrexate [3.9%]), biological drugs (0.8%; n=15), other systemic drugs (4.3%; n=86) and phototherapy (0.2%; n=3). Most patients received these first systemic treatments in monotherapy (98.4% vs 1.7% in combination, being oral corticosteroids the main combination therapy). The median (IQR) duration of first line therapies is presented in Table 2.
Duration of index systemic treatments.
| Variable | N=1995 | |
|---|---|---|
| Duration by treatment type, | n | Median (IQR), days |
| Oral corticosteroids | 1668 | 29.0 (8.8–53.0) |
| Immunosuppressants | 256 | 116.5 (49.0–286.0) |
| Cyclosporine | 106 | 86.0 (33.0–236.0) |
| Azathioprine | 39 | 91.0 (43.0–330.5) |
| Mycophenolate mofetil | 33 | 242.0 (50.0–784.0) |
| Methotrexate | 78 | 133.0 (83.0–387.0) |
| Biologics | 15 | 331.0 (277.5–430.0) |
| Dupilumab | 2 | 327.5 (310.8–344.3) |
| Omalizumab | 4 | 368.0 (299.5–495.5) |
| Rituximab | 9 | 331.0 (201.0–431.0) |
| Other systemics | 86 | 30.0 (30.0–30.0) |
| Immunoglobulins | 72 | 30.0 (30.0–30.0) |
| Interferon gamma | 12 | 165.5 (30.0–310.5) |
| Hydroxychloroquine | 2 | 100.0 (87.0–113.0) |
Note: Phototherapy is not presented in this table as it is based on sessions rather than continuous treatment.
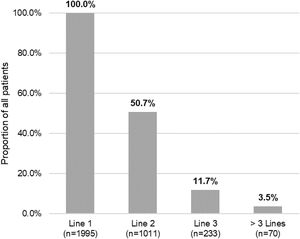
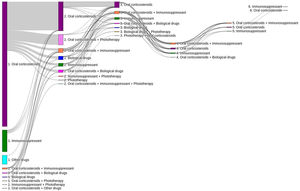
Of the total number of patients included in the study (n=1995), 50.7% (n=1011), 11.7% (n=233) and 3.5% (n=70) required a second, third or fourth systemic treatment line, respectively (Fig. 4). The mean (SD) number of lines of systemic treatment or phototherapy was 1.7 (0.8). As shown in Table 3, the use of oral corticosteroids remained high across the treatment lines (83.6% in Line 1, 86.8% in Line 2, 74.7% in Line 3 and 84.3% in Lines>3), while immunosuppressants were more frequently used in later lines (12.8% in Line 1, 14.5% in Line 2, 38.6% in Line 3, and 30.0% in Lines>3). Biologics were more frequently used in Lines 2 (11.4%) and 3 (13.3%). The percentage of patients who received phototherapy was 11.2%. During the 3-year follow-up period, 12.6% (n=252) of patients received systemic treatment continuously (Table 4). Treatment trajectories were heterogeneous (Fig. 5).
Distribution of systemic treatments/phototherapy by line of therapy.
| Treatment | Line 1N=1995 | Line 2N=1011 | Line 3N=233 | Line>3N=70 | 3 yearsN=1995 |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Oral corticosteroids | 1668 (83.6) | 877 (86.8) | 174 (74.7) | 59 (84.3) | 1706 (85.5) |
| Immunosuppressants | 256 (12.8) | 147 (14.5) | 90 (38.6) | 21 (30.0) | 386 (19.4) |
| Cyclosporine | 106 (5.3) | 52 (5.1) | 15 (6.4) | 0 (0%) | 163 (8.2) |
| Azathioprine | 39 (2.0) | 19 (1.9) | 9 (3.9) | 2 (2.9) | 54 (2.7) |
| Mycophenolate mofetil | 33 (1.7) | 22 (2.2) | 20 (8.6) | 1 (1.4%) | 46 (2.3) |
| Methotrexate | 78 (3.9) | 55 (5.4) | 47 (20.2) | 16 (22.9%) | 146 (7.3) |
| Biologics | 15 (0.8) | 115 (11.4) | 31 (13.3) | 2 (2.9%) | 143 (7.2) |
| Dupilumab | 2 (0.1) | 24 (2.4) | 12 (5.2) | 1 (1.4%) | 34 (1.7) |
| Omalizumab | 4 (0.2) | 35 (3.5) | 5 (2.2) | 1 (1.4%) | 41 (2.1) |
| Rituximab | 9 (0.5) | 56 (5.5) | 14 (6.0) | 0 (0) | 68 (3.4) |
| Other systemics | 86 (4.3) | 0 (0) | 0 (0) | 0 (0) | 86 (4.3) |
| Immunoglobulins | 72 (3.6) | 0 (0) | 0 (0) | 0 (0) | 72 (3.6) |
| Interferon gamma | 12 (0.6) | 0 (0) | 0 (0) | 0 (0) | 12 (0.6) |
| Hydroxycloroquine | 2 (0.1) | 0 (0) | 0 (0) | 0 (0) | 2 (0.1) |
| Phototherapy | 3 (0.2) | 212 (21.0) | 9 (3.9) | 0 (0) | 224 (11.2) |
Lines of systemic treatment/phototherapy during the 3-year follow-up period.
| Variables | N=1995 |
|---|---|
| Number of lines (mean, SD) | 1.7 (0.8) |
| Number of systemic treatment classes received, n (%) | |
| 1 systemic | 1169 (58.6) |
| 2 systemics | 592 (29.7) |
| >2 systemics | 234 (11.7) |
| Patients per treatment line, n (%) | |
| Line 1 | 1995 (100.0) |
| Line 2 | 1011 (50.7) |
| Line 3 | 233 (11.7) |
| >3 Lines | 70 (3.5) |
| Number of patients on continuous systemic treatment excluding oral corticosteroids, n (%) | |
| First year | 374 (18.8) |
| First 2 years | 334 (16.7) |
| 3 years | 252 (12.6) |
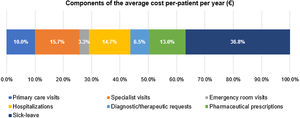
The mean (SD) annual cost per patient was €1278 (€2079), with the following cost components (Fig. 6): €470 (36.8%) sick leave, €200 (15.7%) specialist visits, €187 (14.7%) hospitalization, €167 (13.1%) medication, €128 (10.0%) primary care visits, €83 (6.5%) diagnostic therapeutic requests, and €42 (3.3%) emergency room visits.
The total cost per patient in the 3 years follow up was €3835 (€6238) (Table 5).
Healthcare resource use and associated costs.
| N=1995 | ||||
|---|---|---|---|---|
| Annual | 3 years follow-up | |||
| HRU per patient, mean (SD) | Costs per patient, mean (SD) | HRU per patient, mean (SD) | Costs per patient, mean (SD) | |
| Healthcare, mean (SD) | – | €806 (€1545) | – | €2426 (€4117) |
| Specialist visits | 1.7 (4.5) | €200 (€530) | 5.1 (13.5) | €601 (€1590) |
| Hospitalization | 0.5 (1.1) | €187 (€729) | 1.4 (3.4) | €563 (€2187) |
| Medication | – | €167 (€732) | – | €501 (€2197) |
| Primary care visits | 5.5 (3.1) | €128 (€72) | 16.6 (9.3) | €385 (€215) |
| Diagnostic therapeutic requests | 2.6 (2.4) | €83 (€78) | 7.7 (7.3) | €250 (€235) |
| Emergency room visits | 0.2 (0.8) | €41 (€103) | 0.5 (2.3) | €126 (€310) |
| Non-healthcare, mean (SD) – sick leave | 4.6 (12.4) | €470 (€1253) | 13.9 (37.2) | €1410 (€3760) |
| Total cost per patient, mean (SD) | – | €1278 (€2079) | – | €3835 (€6238) |
The Derma-Atopic Study characterized the clinical, treatment and economic burden of AD in a real-world adult population treated with systemic drugs in Spain. The study provides insights into the patterns of use of systemic treatments over a 3-year period in Spain.
AD patients included in the study were predominantly female, diagnosed in adulthood, with an average of 60 years at systemic treatment initiation, long disease duration and high comorbidity burden (e.g., anxiety, cardiovascular-related diseases). Age at systemic treatment initiation was found to be consistent with other studies based on healthcare databases involving patients in primary and secondary care in Spain 6,13, and outside Spain 20 By contrast, patients selected for participation in primary data collection studies 21,22, where stricter age selection criteria were applied (e.g., adults aged 18–65 years), presented lower mean ages. Coexisting AD-related comorbidities, such as asthma and allergic rhinitis (‘atopic march’), were present in approximately 16% of patients in the current study. This frequency was lower than that reported by Bruin Weller et al., 2020 where a third of patients with moderate–severe disease also had asthma. Concomitant treatments including antibiotics, antihistamines and antidepressants, were highly present in the study population highlighting the significant burden of AD symptoms (e.g., itching) and comorbidities.
Patients analyzed in the study presented considerable use of systemic treatments, with more than half of them requiring at least two lines of treatment in the 3-year study period. First line treatment was mostly based on short courses of oral corticosteroids, which also remained consistently present across all treatment lines (both in monotherapy and in combination). The high use of oral corticosteroids versus other alternatives such as immunosuppressants, may be explained by the contribution of patients from primary care practices in addition to secondary care, where a higher use of immunosuppressants may be expected. Patients who received immunosuppressants or biologics as first line, remained on treatment for longer periods (average of 117 and 331 days, respectively). The use of immunosuppressants (mostly consisting of cyclosporine and methotrexate, the latter used off-label) increased as patients advanced in the treatment pathway, in alignment with recommendations in current treatment guidelines1. Interestingly, although guidelines limit the use of intravenous immunoglobulin to children with refractory disease, about 4% of patients in the study and 20 of the 240 included patients in the Pereyra-Rodriguez12 study reported the use of this treatment in adults. As this study provides an overview of the AD treatment landscape between 2013 and 2019, the use of dupilumab (reimbursed in 2020 in Spain)23 and that of other biologics (off-label) was scarce. The availability of biological drugs and Janus kinase inhibitors (JAKi) is expected to result in a change of treatment paradigm in the moderate–severe AD setting24, requiring further characterization of treatment pathways in the upcoming years.
Variability of clinical practice and treatment trajectories observed in the study confirms the heterogeneous nature of the disease which requires individualized treatment strategies in routine practice13,25,26. About 20% of patients in the study received systemic treatments continuously over a year and 13% over a 3-year period. These findings suggest that, while some patients may present acute flare-driven disease requiring short term pharmacological interventions to relieve symptoms, others develop a chronic disease course with persistent skin lesions and intense pruritus 27. However, the lack of data on severity scores, patient-reported outcomes (PROs) and symptoms, limited the interpretation of these results. Additionally, further research to describe the type of physicians initiating and prescribing systemic treatments (e.g., general practitioner versus dermatologist) and referral patterns for moderate–severe AD patients in the real world is warranted.
AD poses a significant economic burden for the healthcare system, patients and their families, especially in its most severe forms1,2,28. The present study estimated the cost of moderate–severe adult AD in €1278 per patient per year (healthcare costs: 63%; indirect costs: 37%). A recent study conducted in Catalonia reported a higher unit cost, which raised up to €3397 for severe and €2111 for moderate patients with a 75.5% for healthcare costs and 24.5% for indirect costs 6. In both studies indirect costs based on productivity losses were heavily represented, finding that aligns with existing evidence suggesting a significant impact of AD on work productivity 29.
This study presents some limitations. The possible inaccuracy of the diagnostic coding for AD and other comorbidities, or the lack of data on socio-economic level and clinical status, contributing regions or hospitals which remain confidential, therapeutic adherence, phenotypes, etc. in the source database to further characterize the sample may constitute potential limitations of the study. Also, there may be an over representation of older AD cases in the study which may hinder comparison with studies using different data sources. The validation and representativeness of the BIG-PAC database was previously explored, however, the analysis lacked socio-economic and educational level, and the type of population covered (urban/rural) 15. While some uncertainty remains as to whether the results obtained in the present study can be generalized to the general population in Spain, the published evidence using the BIG-PAC database indicates that it is appropriate to conduct studies in the real world setting for diverse conditions including AD in Spain13,30–33. Additionally, the BIG-PAC database is registered in the ENCePP inventory as a data source eligible for conducting pharmacoepidemiology and pharmacovigilance studies in Europe. Treatment and PROs and occurrence of flares were not recorded hindering the clinical interpretation of results. The study attempted to select patients initiating a systemic drug or phototherapy for the first time to treat AD in their adult life's. To that purpose, patients with a prior record of systemic use or phototherapy in the 2 years prior to the index date were excluded. However, prior systemic use beyond those 2 years cannot be excluded. Additionally, costs were based on resources identified using proxies for AD-related in the absence of actual codes linked to each resource. Therefore, it is possible that some resource types are overestimated.
ConclusionsThis study described a real-world population of adults with AD treated with systemic drugs in Spain. Results suggest a high comorbidity and economic burden for the healthcare system, patients and their families. While variability in treatment patterns was observed, short courses of oral corticosteroids remained as the main treatment option followed by immunosuppressants, several used off-label. This highlights the need for new systemic treatments indicated for use in AD.
FundingThis study was funded by Eli Lilly and Company.
Conflict of interestEsther Serra has received fees for acting as a scientific advisor and speaker for Eli Lilly and Company. Esther Artime, Silvia Díaz, and Teresa Huete are employees of and minor shareholders in Eli Lilly and Company. Ignacio Hernández, Laura LLedo and Antoni Sicras are employees of and minor shareholders in Atrys Health S.A. in Spain.