There is no consensus on the response rates of secukinumab, as findings from phase III trials differ from those reported in clinical-practice studies. This study aimed to assess the mid-term safety and efficacy profile of secukinumab in patients with moderate-to-severe hidradenitis suppurativa (HS).
MethodsThis retrospective, multicenter study included patients with moderate-to-severe HS treated with secukinumab (300mg every four weeks) between 2020 and 2024. Eligible patients were aged ≥18 years, with a clinical diagnosis of moderate-to-severe HS, and a minimum follow-up of 24 weeks. Key exclusions included patients treated with nonstandard secukinumab dosing regimens or those with insufficient clinical data. The primary endpoints were achievement of HiSCR50 (≥50% reduction in the combined count of nodules and abscesses) at weeks 16 and 24). Secondary endpoints included achieving a ≥55% reduction in the International HS Severity Score (IHS4-55) and adverse events.
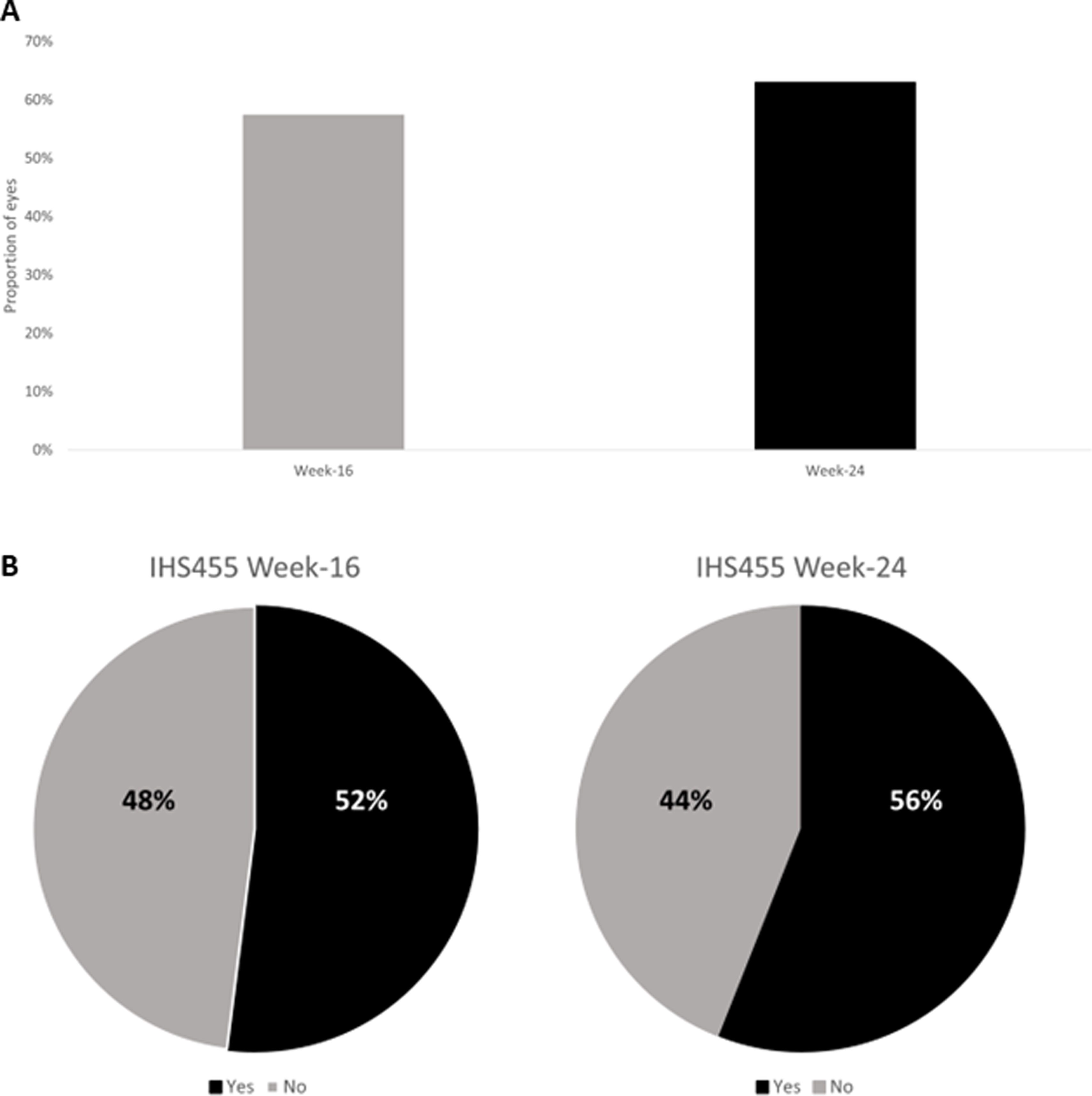
ResultsA total of 263 patients (49.4%, women; 50.6%, men; mean age, 41.8±13.2 years) were included. The most common HS phenotype was mixed (49.4%), and 55.1% had Hurley stage III disease. At weeks 16 and 24, a total of 57.4% and 63.6% of patients achieved HiSCR50, respectively. The mean IHS4 score dropped significantly from 16.7±10.0 at baseline to 7.6±5.6 at week 24 (p<0.0001). At weeks 16 and 24, 52% and 56% of patients achieved an IHS4-55 response. Secukinumab was discontinued in 14.5% of patients because of lack of efficacy or adverse events.
ConclusionsSecukinumab demonstrated a significant safety and efficacy profile for patients with moderate-to-severe in a real-world setting, with better outcomes than those reported in earlier clinical trials. Response evaluation at both week 16 and 24 is crucial due to variations in treatment effectiveness.
HS (HS) is a persistent, relapsing, inflammatory dermatological condition originating from pilosebaceous units, predominantly impacting intertriginous regions, and frequently correlated with multiple systemic comorbidities.1 Due to its chronicity and recurrent episodes, HS significantly impacts the patients’ quality of life, profoundly affecting social, occupational, and psychological dimensions.2 In Europe, the reported prevalence of HS fluctuates from 1% to 4% across various studies, attributable to differences in study populations and methodologies3; while epidemiological data from American surveys report prevalences between 0.05% and 0.20%.4,5
HS is clinically marked by recurrent episodes of neutrophilic inflammation. The inflammation initiates in the hair follicles, leading to the formation of painful nodules and abscesses, which, in advanced stages, develop into pus-discharging tunnels and extensive scarring.1,3
Although the pathogenesis of HS has not been fully understood, it is a multifactorial disease resulting from a combination of genetic, environmental, and immunologic factors.1,3,6–8
The initial pathophysiological events in HS are thought to be driven by the release of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) following early follicular occlusion and bacterial overgrowth. These events activate the inflammasome, leading to the secretion of Interleukin (IL)-1β, primarily by tissue macrophages, and subsequent release of downstream cytokines, including IL-17 and TNFα (6-8). Altered Toll-like receptor (TLR) signaling in macrophages and dendritic cells (DCs), which are the predominant cells in HS lesions, results in the elevated production of these cytokines, which triggers the activation of DCs, which in turn secrete IL-23, promoting the polarization of Th17 cells. IL-17-producing T helper cells have been observed to infiltrate the dermis in chronic HS lesions.6–8
HS treatment depends on disease severity and individual impact, involving topical, systemic, surgical, and combined approaches.9 Topical therapy is preferred in early stages, while systemic therapies, such as antibiotics, anti-inflammatory agents, immunosuppressants, botulinum toxin, isotretinoin, and antiandrogens are used for more severe cases.6,10
The introduction of biologics has transformed the management of moderate-to-severe HS. For years, adalimumab (a TNF-α inhibitor) was the only approved biologic.11,12 Recently, anti-IL-17 biologics, such as secukinumab, ixekizumab, brodalumab have emerged as options for patients unresponsive to other therapies.13–15
Data from 2 double-blind, randomized phase III clinical trials (SUNSHINE and SUNRISE) have demonstrated the safety and efficacy profile of secukinumab for treating moderate-to-severe HS vs placebo, with 42–48% of patients achieving HS Clinical Response (HiSCR).16–18 Additionally, different clinical-practice studies have shown that secukinumab may be effective in treating HS, although the proportion of treatment-responders was variable.19–27
The current study aimed to assess the mid-term safety and efficacy profile of secukinumab in patients with moderate-to-severe HS.
MethodsStudy designWe conducted a retrospective and multicenter study under real-world conditions, including consecutive HS patients with moderate-to-severe HS28 who underwent treatment with secukinumab from 2020 through 2024. The study ensured a minimum follow-up period of 24 weeks, with a maintenance dose of 300mg administered every 4 weeks.
The study protocol was approved from IIS La Fe Ethics Committee (Valencia, Spain), which waived the requirement for informed consent to conduct the study. However, all participants gave their prior written informed consent before receiving secukinumab. This study fully complied with the Good Clinical Practice/International Council for Harmonization Guidelines, the Declaration of Helsinki, and all applicable country-specific regulations governing clinical research, prioritizing whichever provided greater protection to the individual. All identifying information was encrypted or withdrawn to ensure participant anonymity.
Study populationPatients aged ≥18 years old with a clinical diagnosis of moderate-to-severe HS, who underwent treatment with secukinumab, with a maintenance dose of 300mg administered every 4 weeks, and had a minimum 24-week follow-up. We excluded those patients with other secukinumab posology and cases with a lack of the data needed to analyze the therapeutic response.
Patients were required to have a confirmed diagnosis of HS for at least 6 months prior to study initiation, in accordance with the inclusion criteria defined in the SUNNY trials.16–18
OutcomesThe primary endpoints were HiSCR50 (defined as the proportion of patients who achieved at least a 50% reduction in nodule and abscess count) at week 16 and week 24.
Secondary endpoints were the proportion of patients who achieved a ≥55% reduction of the International HS Severity Scoring System (IHS4-55) score29 and the safety profile (in terms of treatment-related adverse events).
Clinical phenotypes of HS were defined according to Martorell et al. classification.30
Pain was determined according to the Numerical Rating Scale (NRS)9; however, due to the limited number of patients with available data (n=12), this variable was excluded from the statistical analysis.
Statistical analysisA standard statistical analysis was performed using SPSS Statistical Software version 28 (IBM SPSS Statistics, IL, United States).
Descriptive statistics, including number (percentage) and mean±standard deviation (SD), were applied as appropriate.
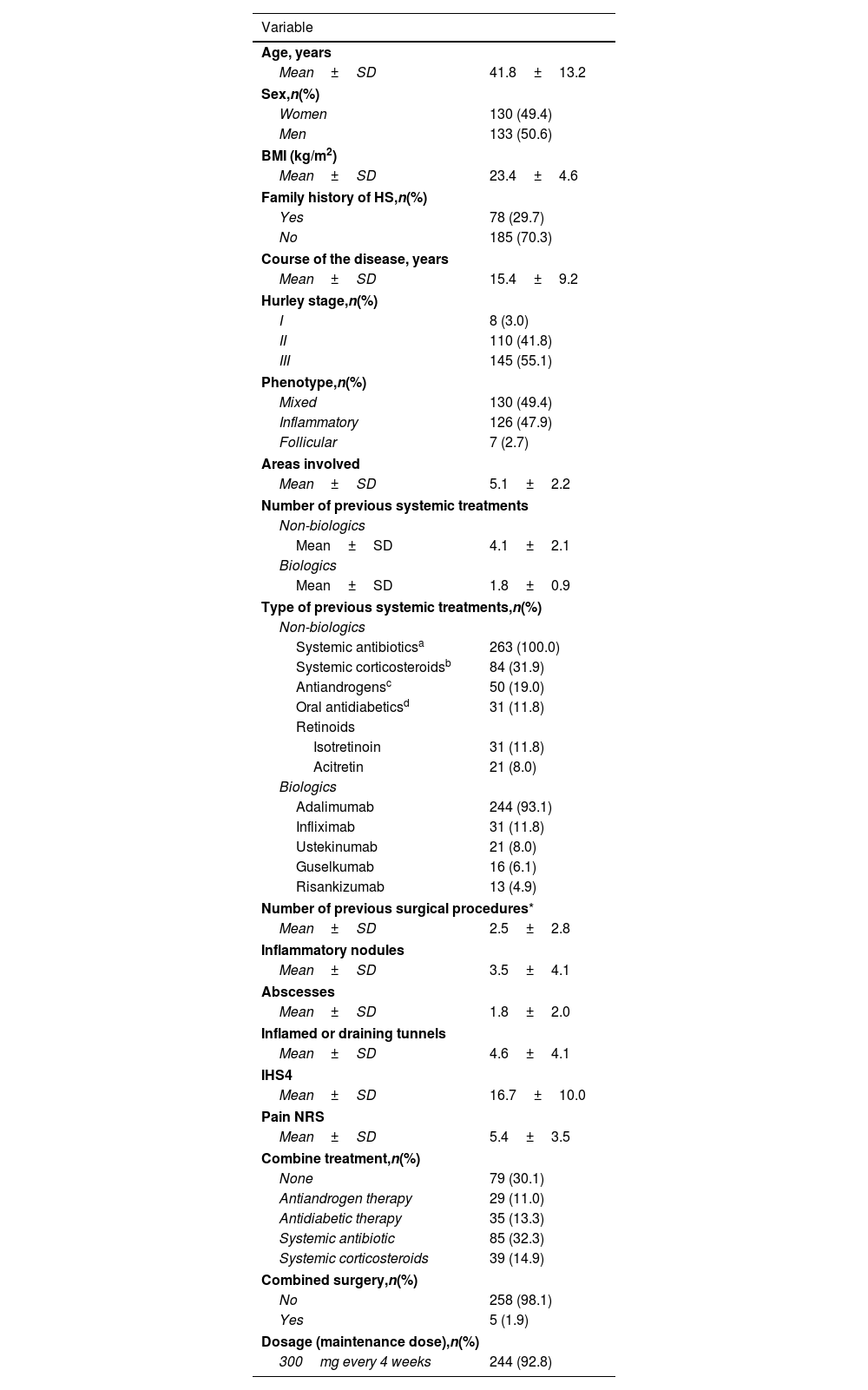
ResultsBaseline demographic and clinical characteristicsA total of 263 patients, 130 (49.4%) women and 133 (50.6%) men, were included in the study. Mean age was 41.8±13.2 years.
The most prevalent HS phenotype was the mixed type, observed in 130 (49.4%) patients, followed by the inflammatory phenotype in 47.9% of patients, and the follicular phenotype in 2.7%.
Regarding HS severity, a total of 145 (55.1%) patients exhibited Hurley stage III disease; 110 (41.8%) patients, Hurley stage II disease; and 8 (3.0%) patients, Hurley stage I disease.
The mean ISHA score was 16.7±10.0. The mean number of previous systemic treatment were 4.1±2.1 and 1.8±0.9 drugs for the non-biologic and biologic therapies, respectively. Of the 263 patients included in the study, 18 (6.8%) were biologic-naïve at the time of treatment initiation. The mean number of previous surgical procedures, excluding incision and drainage was 2.5±2.8 procedures.
The main demographic and clinical characteristics are shown in Table 1.
Key demographic and clinical features of the study cohort.
| Variable | |
|---|---|
| Age, years | |
| Mean±SD | 41.8±13.2 |
| Sex,n(%) | |
| Women | 130 (49.4) |
| Men | 133 (50.6) |
| BMI (kg/m2) | |
| Mean±SD | 23.4±4.6 |
| Family history of HS,n(%) | |
| Yes | 78 (29.7) |
| No | 185 (70.3) |
| Course of the disease, years | |
| Mean±SD | 15.4±9.2 |
| Hurley stage,n(%) | |
| I | 8 (3.0) |
| II | 110 (41.8) |
| III | 145 (55.1) |
| Phenotype,n(%) | |
| Mixed | 130 (49.4) |
| Inflammatory | 126 (47.9) |
| Follicular | 7 (2.7) |
| Areas involved | |
| Mean±SD | 5.1±2.2 |
| Number of previous systemic treatments | |
| Non-biologics | |
| Mean±SD | 4.1±2.1 |
| Biologics | |
| Mean±SD | 1.8±0.9 |
| Type of previous systemic treatments,n(%) | |
| Non-biologics | |
| Systemic antibioticsa | 263 (100.0) |
| Systemic corticosteroidsb | 84 (31.9) |
| Antiandrogensc | 50 (19.0) |
| Oral antidiabeticsd | 31 (11.8) |
| Retinoids | |
| Isotretinoin | 31 (11.8) |
| Acitretin | 21 (8.0) |
| Biologics | |
| Adalimumab | 244 (93.1) |
| Infliximab | 31 (11.8) |
| Ustekinumab | 21 (8.0) |
| Guselkumab | 16 (6.1) |
| Risankizumab | 13 (4.9) |
| Number of previous surgical procedures* | |
| Mean±SD | 2.5±2.8 |
| Inflammatory nodules | |
| Mean±SD | 3.5±4.1 |
| Abscesses | |
| Mean±SD | 1.8±2.0 |
| Inflamed or draining tunnels | |
| Mean±SD | 4.6±4.1 |
| IHS4 | |
| Mean±SD | 16.7±10.0 |
| Pain NRS | |
| Mean±SD | 5.4±3.5 |
| Combine treatment,n(%) | |
| None | 79 (30.1) |
| Antiandrogen therapy | 29 (11.0) |
| Antidiabetic therapy | 35 (13.3) |
| Systemic antibiotic | 85 (32.3) |
| Systemic corticosteroids | 39 (14.9) |
| Combined surgery,n(%) | |
| No | 258 (98.1) |
| Yes | 5 (1.9) |
| Dosage (maintenance dose),n(%) | |
| 300mg every 4 weeks | 244 (92.8) |
SD: standard deviation; BMI: body mass index; HS: HS; IHS4: International HS Severity Scoring System; NRS: Numerical Rating Scale.
The proportion of patients who achieved a HiSCR50 at week-16 and at week-24 of secukinumab treatment were 57.4% and 63.6%, respectively (Fig. 1A).
International HS Severity Score System (IHS4-55)The mean IHS4 score significantly dropped from 16.7±10.0 points at baseline to 10.1±5.2 points at week 16 and 7.6±5.6 points at week 24, with both reductions being statistically significant (p<0.0001 vs baseline).
At week 16 and 24 of secukinumab treatment, 52% and 56% of patients reached an IHS455 (Fig. 1B).
Compared with the administration of secukinumab alone, the combination therapy showed better results, regardless of the scale or time of measurement. At week 16, the proportion of patients achieving HiSCR50 was significantly higher in the combination therapy group (62.2%) vs those on secukinumab monotherapy (52.2%) (p<0.05). This difference persisted and slightly increased by week 24, with response rates of 66.7% vs 55.5%, respectively (p<0.05).
A time-restricted subanalysis revealed statistically significant differences in treatment response based on baseline disease severity and duration. Patients with moderate disease (Hurley II) showed higher response rates vs those with severe disease (Hurley III), achieving HiSCR50 in 62.2% and 65.7% of cases at weeks 16 and 24, respectively, and IHS4-55 in 68.1% at week 24. In contrast, Hurley III patients had lower rates: 48.3% (HiSCR50, week 16), 51.1% (week 24), and 52.3% (IHS4-55) (p<0.05).
Similarly, shorter courses of the disease (<5 years) were associated with better outcomes: HiSCR50 responses were 63.3% at week 16 and 68.4% at week 24, with 71.2% reaching IHS4-55. Patients with longer disease duration (>5 years) had lower response rates (51.2%, 53.4%, and 55.5%, respectively; p<0.05). These findings underscore the influence of disease stage and chronicity on treatment efficacy.
Safety and treatment withdrawalThroughout follow-up, 38 (14.5%) patients withdrew secukinumab due to lack of response (n=33; 12.6%); oral candidiasis (n=1, 0.4%) psoriasis worsening (n=2; 0.8%); ulcerous colitis flare (n=1; 0.4%); and Crohn's disease flare (n=1; 0.4%).
DiscussionAccording to the results of the current real-world study, we concluded that in clinical practice secukinumab demonstrates to be a safe and effective treatment for patients with HS refractory to conventional systemic therapy. Key highlights include the high proportion of patients who achieved an IHS4-55 and HiSCR at week-16 and week-24 of treatment, as well as the low rate of treatment discontinuation (12.6%).
The Sunny trials (Sunrise and Sunshine)16–18 were 2 randomized control trials that assessed the safety and efficacy profile of secukinumab in patients with moderated-to-severe HS over a period of 52 weeks. The primary endpoint was the proportion of patients achieving HiSCR50, which ranged from 42% (Sunrise) to 46% (Sunshine) in the treatment groups.
Although HiSCR is a tool widely used in clinical practice for assessing the reduction of inflammatory nodules and abscesses, it does not include the reduction of draining tunnels in its evaluation.11. Recently, the IHS4-55 tool has been introduced for evaluating the efficacy of HS treatments.28,31
At week 16, 57.4% of patients achieved HiSCR50. In the same line, at week 16 up to 52% of patients reached an IHS455 (Fig. 1B).
The results of our study suggested slightly improved outcomes vs those reported by the “Sunny trials.” Specifically, the proportion of patients achieving HiSCR50 was 57.4% at week 16 (vs 42% in the Sunrise and 46% in the Sunshine) and 63.6% at week 24 (vs 56% in the Sunny).16,17
The slightly better outcomes observed in our study may be attributed to the fact that 69.9% of the sample received combination therapy. Clinical trials on HS typically exclude treatments aimed at managing comorbidities that could potentially influence patient outcomes.16–18 In contrast, daily clinical practice and current clinical guidelines underscore the importance of addressing comorbidities, as their effective management positively impacts the control of HS.32–34
Although randomized clinical trials (RCTs) represent the highest level of clinical evidence,35 they have limitations that may reduce their clinical validity and applicability to certain population groups. Clinical-practice data can offer valuable insights into treatment efficacy across diverse patient subgroups in clinical practice.36,37
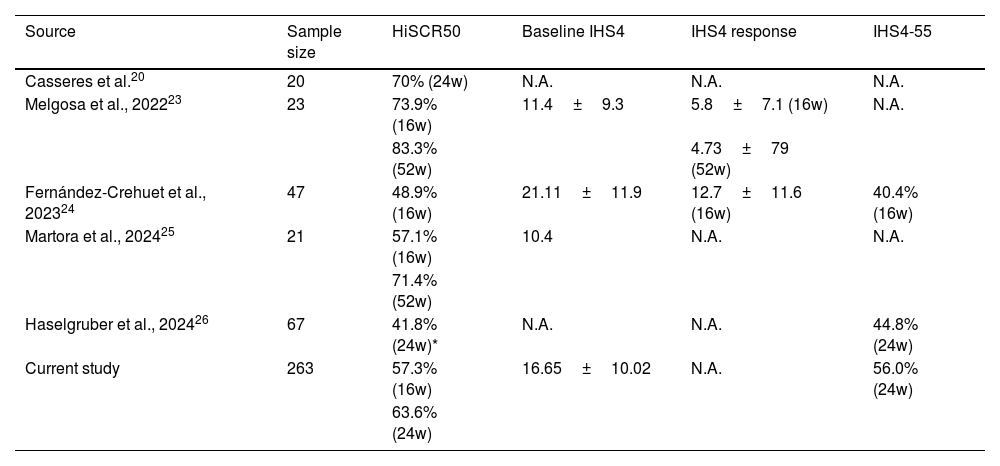
Other studies, conducted on daily clinical-practice conditions, have assessed the effectiveness of secukinumab in HS, predominantly using HiSCR as the primary outcome measure, with HiSCR rates ranging from 46% to 83.6% at 16–52-week follow-ups.19–27 However, these studies often involved limited samples (Table 2).
A comparison of the clinical outcomes between the current study and the available real-world evidence.
| Source | Sample size | HiSCR50 | Baseline IHS4 | IHS4 response | IHS4-55 |
|---|---|---|---|---|---|
| Casseres et al.20 | 20 | 70% (24w) | N.A. | N.A. | N.A. |
| Melgosa et al., 202223 | 23 | 73.9% (16w) | 11.4±9.3 | 5.8±7.1 (16w) | N.A. |
| 83.3% (52w) | 4.73±79 (52w) | ||||
| Fernández-Crehuet et al., 202324 | 47 | 48.9% (16w) | 21.11±11.9 | 12.7±11.6 (16w) | 40.4% (16w) |
| Martora et al., 202425 | 21 | 57.1% (16w) | 10.4 | N.A. | N.A. |
| 71.4% (52w) | |||||
| Haselgruber et al., 202426 | 67 | 41.8% (24w)* | N.A. | N.A. | 44.8% (24w) |
| Current study | 263 | 57.3% (16w) | 16.65±10.02 | N.A. | 56.0% (24w) |
| 63.6% (24w) |
N.A.: not available; w: week; m: minute; HiSCR: hidradenitis suppurativa clinical response; IHS4-55: proportion of patients who achieved a 55% reduction of the International HS Severity Scoring System (IHS4) score.
Casseres et al.21 found that 65% (13/20) of patients achieved HiSCR at week 12. Similarly, Melgosa et al.24 followed 23 patients and reported that 73.9% (17/23) achieved HiSCR at week 16, with sustained results of 71.4% (15/21) at week 24, 71.4% (10/14) at week 36, and 83.3% (10/12) at week 52 of secukinumab treatment. Fernandez-Crehuet et al.25 reported that 48.9% of patients achieved HiSCR at week 16, while Martora et al.26 found that 57.1% (8/14) of patients achieved HiSCR at week 16, with 71.4% (10/14) reaching HiSCR by week 52.
There is ongoing debate regarding the optimal time point for assessing therapeutic response to biologic agents, with week 24 generally considered the most informative in clinical practice. In our study, we observed an increased response at this time point, with 63.6% of patients achieving HiSCR50 and up to 56% achieving IHS4-55. These findings contrast with those reported by Haselgruber et al.,27 in a retrospective study of 67 patients, where 41.79% (28/67) achieved HiSCR and 44.78% (30/67) achieved IHS4-55 at week 24.
To the best of our knowledge, the current study, which includes 263 patients, is the largest clinical-practice analysis of secukinumab for HS conducted thus far.
Compared with published evidence, both clinical trials and clinical-practice studies, the HiSCR rate in our study is in the upper range of these studies. Nevertheless, it must be considered that the sample of this study presented a high proportion of patients at the Hurley III stage (indeed 97% of patients were either stage II or III), a long disease duration (15.4±9.2 years), and that patients presented previous exposure to biologic drugs (mean, 1.8±0.9 drugs).
This study has limitations that should be considered when evaluating its results. Firs, its retrospective design introduces potential confounding variables and inherent biases. Nevertheless, a key strength of this study is its execution within clinical settings, allowing for the inclusion and analysis of patient scenarios that extend beyond those typically assessed in controlled clinical trials. Secondly, its limited follow-up duration. A longer follow-up period might provide more comprehensive insights. Important strengths of this study include its multicenter design and the high number of patients included in it.
ConclusionsThe results of this multicenter real-world study indicate that secukinumab can be considered a safe and effective treatment option for patients with moderate-to-severe HS who have failed other therapies, even with higher results vs the previous data published in the literature from the Sunny clinical trials. Special consideration would be made in the best moment to evaluate the response to medical therapy due to the differences between week 16 and 24.
FundingMedical writing service has been supported by the AEDV Spanish Group of Psoriasis.
Conflicts of interestAntonio Martorell declared to have received honoraria and/or travel grants and/or acted as an advisory board member for Novartis, AbbVie, Janssen Cilag, UCB, Lilly, LEO Pharma, L’Oreal, Sanofi, Boehringer Ingelheim, Almirall, Bristol Myers Squib and Amgen. Moreover, he has worked as a principal investigator in clinical trials supported by AbbVie, UCB, Jansen, Bristol Myers Squibb, Lilly, Galderma, Sanofi, and Novartis.
Francisco Javier Melgosa Ramos declared to have received honoraria and/or travel grants and/or acted as an advisory board member for Novartis, Abbvie, Janssen Cilag, UCB, Lilly, LEO Pharma, L’Oreal, Sanofi, Almirall and Amgen. The remaining authors declared no conflicts of interest whatsoever.
Medical writing and Editorial assistant services have been provided by Ciencia y Deporte S.L.