Clinical trials have validated the use of nivolumab and pembrolizumab as adjuvant therapies regarding relapse-free survival in patients with resected stage III and IV melanoma. Evidence in real-world patients is currently limited.
Material and methodThe CADIM trial (characterization of adjuvant immunotherapy in melanoma patients) recruited a total of 81 patients with resected stage III and IV melanoma on nivolumab or pembrolizumab as adjuvant therapy from February 2018 to December 2022.
ResultsThe stage distribution rate was 81.5% (n=71) for stage III, while 15 patients (18.5%) had resected stage IV. Among stage III patients, 38 were stage IIIC (46.9%). With a median follow-up of 22.8 months, the relapse-free survival in the intention-to-treat population was 84% at one year and 81.5% at 2 years. The overall survival rate was 99% at one year and 91.4% at 2 years. Grade 3–4 treatment-related adverse events were reported in 12.3% of the patients.
ConclusionsThis study shows the results of resected stage III and IV melanoma patients on adjuvant therapy with anti-PD-1, and eventually confirmed the safety and efficacy profile described by clinical trials. Comparing clinical trial data with real-world evidence is necessary for a more practical, reliable, and accessible use of these drugs.
Los ensayos clínicos han validado el uso de nivolumab y pembrolizumab en adyuvancia, en términos de supervivencia libre de recaída, en los pacientes con melanoma resecado en estadios III y IV. La evidencia en los pacientes en la práctica clínica es limitada.
Material y métodoPara el estudio Caracterización de la ADyuvancia con Inmunoterapia en pacientes con Melanoma (CADIM) se seleccionó de febrero de 2018 a diciembre de 2022, a 81 pacientes con melanoma en estadios III y IV resecados, que recibieron nivolumab o pembrolizumab en adyuvancia.
ResultadosLa distribución por estadios fue del 81,5% (n=71) para estadios III, mientras que 15 pacientes (18,5%) presentaban un estadio IV resecado. Entre los estadios III, 38 pacientes eran IIIC (46,9%). Con una mediana de seguimiento de 22,8 meses, la supervivencia libre de recaída en la población con intención de tratar fue del 84% a un año y del 81,5% a 2 años. La supervivencia global fue del 99% a un año y del 91,4% a 2 años. Los efectos adversos relacionados con el tratamiento de grado 3-4 aparecieron en el 12,3% de los pacientes.
ConclusionesEste estudio presenta los resultados de los pacientes con melanoma en estadios III y IV resecados, tratados con anti-PD-1 en adyuvancia. En él se confirma la eficacia y la seguridad observada en los ensayos clínicos. Contrastar los datos de los ensayos clínicos con la evidencia encontrada en la práctica clínica es necesario para hacer más útil, confiable y cercano el uso de estos fármacos.
Both immunotherapy and targeted therapy have shown efficacy in patients with metastatic melanoma, which has altered the current landscape for these patients1–3 and later demonstrated benefits in the adjuvant setting for high-risk patients.3,4 The CheckMate 238 and Keynote 054 clinical trials are phase III studies that included patients with resected melanoma at stages (AJCC 7th edition) IIIB, IIIC, IV, and IIIA (with lymph node involvement>1mm), IIIB, and IIIC, respectively. Although the control groups varied (high-dose ipilimumab for CheckMate 238 and placebo for Keynote 054), the 2 drugs (nivolumab and pembrolizumab) proved beneficial in relapse-free survival (RFS): 50% for nivolumab and 39% for ipilimumab at 5 years, and 59.8% for pembrolizumab vs 41.4% in the placebo group at 5 years. Currently, the RFS rates after the AJCC 8th edition classification, with nivolumab at 5 years, is 66% (49% with ipilimumab for stage IIIB), 44% vs 39% for stage IIIC, and 28% vs 0% for stage IIID.5 The distant metastasis-free survival for pembrolizumab at 3.5 years is 80.8% in stage IIIA vs 70.8% with placebo, 68.1% vs 51% in stage IIIB, and 55.8% vs 39.2% in stage IIIC (AJCC 7th edition).5
Grade 3–4 treatment-related adverse events occurred in 14.4% of patients with nivolumab and 14.5% with pembrolizumab, with a discontinuation rate of 7.7% and 14.4%, respectively.6,7 Quality of life was not altered by either nivolumab or pembrolizumab.7,8
The results in routine clinical practice may differ from those of the trials. Registry studies were conducted using the 7th edition of the AJCC staging system, and all patients underwent lymphadenectomy after a positive sentinel lymph node biopsy (SLNB). However, the 8th edition of the AJCC is currently being used,9 and following the results of the MLST II and DeCOG studies,10,11 there is minimal use of lymphadenectomy. Therefore, it is useful to obtain data on the use of adjuvant therapy in melanoma patients in the routine clinical practice.
These data are needed to validate those obtained in trials, although there are few studies in the adjuvant setting. De Meza et al. reported a clinical practice analysis of 641 patients in stages III or IV on nivolumab and pembrolizumab after lymphadenectomy and reclassification to the 8th edition of the AJCC. After one year, the RFS rate was 70.6%, and grade≥3 toxicity appeared in 18% of patients.12 In a different study by Hofmann et al., 30 patients on nivolumab had a 40% recurrence rate (half occurring during the adjuvant phase), and a 16.7% toxicity rate at grade 3–4.13 The German group reported the results of a retrospective analysis of 100 patients on immunotherapy, with an estimated 1-year RFS rate of 64.8%, 16% grade≥3 toxicity, and a 22% adverse event-related discontinuation rate.14 More recently, results from a large cohort in Germany, Austria, and Switzerland with 1198 patients on anti-PD1 and anti-BRAF/anti-MEK agents showed a 1-year RFS rate of 74.1%, with 12.3% of patients discontinuing treatment due to adverse effects.15 Additionally, data were reported from an Italian expanded access cohort, with 1-year RFS and overall survival rates of 76.6% and 93.8%, respectively.16
The CADIM study (characterization of adjuvant immunotherapy in melanoma patients) aims to analyze the characteristics of melanoma patients on adjuvant immunotherapy in the Autonomous Community of the Region of Murcia (Spain) outside the clinical trial setting. We present the updated final data from the 81 patients treated at Hospital Clínico Universitario Virgen de la Arrixaca (HCUVA) in the city of Murcia and Complejo Hospitalario de Cartagena (CHC) (Murcia, Spain).
MethodsStudy plan and treatment schemeFrom February 2018 to December 2022, a total of 81 patients with resected melanoma at stages III or IV received nivolumab (3mg/kg IV every 2 weeks) or pembrolizumab (3mg/kg IV every 3 weeks) for up to 1 year as part of the routine clinical practice outside the clinical trial setting, including early discontinuations due to disease recurrence, unacceptable toxicity, or withdrawal of consent.
All patients signed the usual informed consent used in the centers where the study was being conducted. The study was approved by Hospital Virgen de la Arrixaca Clinical Research Ethics Committee.
Inclusion criteria:
- a)
Age≥18 years.
- b)
Resected melanoma at stage III (with lymph node involvement≥1mm) or IV according to the AJCC 8th edition. Mucosal melanomas were staged using the classification of Cui et al., based on the AJCC 8th edition.17
- c)
Includes acral melanomas, mucosal melanomas, and melanomas of unknown primary.
- d)
Initiation of adjuvant therapy≤12 weeks after the last surgery.
- e)
No prior systemic treatments or radiotherapy.
- f)
Disease-free status documented before starting adjuvant therapy, via physical examination and imaging modalities used in the routine clinical practice. Follow-up was conducted using the same techniques every 3–4 months.
- g)
ECOG≤2.
- h)
Normal lab test results before starting treatment (2 weeks).
- i)
Absence of brain metastasis.
Exclusion criteria:
- a)
Other active cancers within the last 3 years, except for skin cancers (non-melanoma) or in situ carcinomas.
- b)
Known and active autoimmune diseases, except for those requiring hormonal replacement therapy or skin changes without systemic treatment.
- c)
Ocular melanoma.
Statistical analysis was performed using the Statistical Package for the Social Sciences version 20. A descriptive analysis of the data was conducted. Categorical variables were expressed as absolute values and percentages, and quantitative variables as medians and intervals. Survival times were calculated using the Kaplan–Meier method. RFS was calculated from the start date of the first cycle until relapse, whether locoregional or distant, or death (related or not to melanoma), or until the last follow-up censoring. Overall survival was calculated from the start date of treatment until death or the censoring date at the last follow-up.
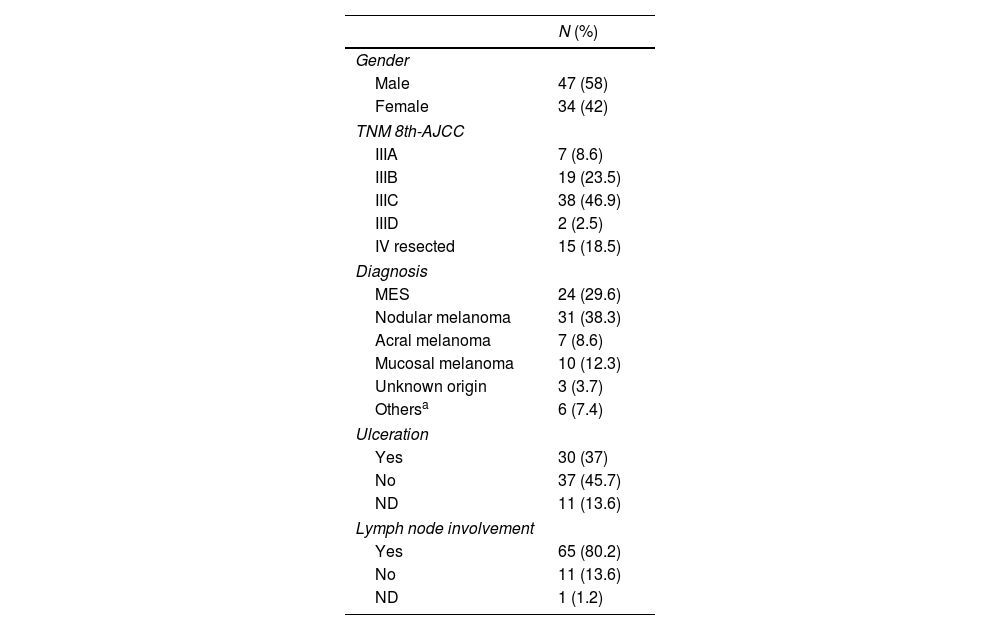
ResultsStudy populationThe characteristics of the patients are summarized in Table 1. A total of 81 patients were included, with a median age of 58 years (18–82), most being men (58%). The most common subtype of melanoma was cutaneous (76.5%), followed by mucosal melanoma (12.3%). Regarding staging, based on the AJCC 8th edition TNM classification, 81.5% (n=66, 7 of them had mucosal melanoma) were stage III, most were stage IIIC (n=38; 46.9%), and 15 patients (18.5%) were resected stage IV. Out of the 10 patients with mucosal melanoma, 7 were stage III, and the remaining 3 were stage IV resected.
Patient characteristics.
| N (%) | |
|---|---|
| Gender | |
| Male | 47 (58) |
| Female | 34 (42) |
| TNM 8th-AJCC | |
| IIIA | 7 (8.6) |
| IIIB | 19 (23.5) |
| IIIC | 38 (46.9) |
| IIID | 2 (2.5) |
| IV resected | 15 (18.5) |
| Diagnosis | |
| MES | 24 (29.6) |
| Nodular melanoma | 31 (38.3) |
| Acral melanoma | 7 (8.6) |
| Mucosal melanoma | 10 (12.3) |
| Unknown origin | 3 (3.7) |
| Othersa | 6 (7.4) |
| Ulceration | |
| Yes | 30 (37) |
| No | 37 (45.7) |
| ND | 11 (13.6) |
| Lymph node involvement | |
| Yes | 65 (80.2) |
| No | 11 (13.6) |
| ND | 1 (1.2) |
Most patients were BRAF wild-type (40.7%), 28.4% were BRAF-mutated, and the BRAF status was unknown in 30.9% of cases.
SLNB was performed in 79% of the patients, and 45.7% underwent lymphadenectomy.
Relapse-free survival and overall survivalAs of the data cutoff in December 2022, the median follow-up was 22.8 months.
RFS was 45.6 months (95%CI, 40.2–51.1) without reaching the median, with 84% of patients free of relapse at one year and 81.5% at two years.
The RFS by stage was 18.4 months (95%CI, 0–40.2), 19.1 months (95%CI, 0–38.6), 19.9 months (95%CI, 10.3–29.4), 3.6 months (single value), and 8.3 months (95%CI, 0–16.9) for stages IIIA, IIIB, IIIC, IIID, and IV, respectively.
The median overall survival was 49.3 months (95%CI, 44.3–54.2) without reaching the median, with 12 patients deceased at the data cutoff. A total of 98% were alive 1 year after treatment initiation and 91.4% at 2 years.
The median overall survival by stage was 18.4 months (95%CI, 0–40.2), 25.1 months (95%CI, 15.6–34.6), 23.2 months (95%CI, 18.7–27.6), 19.9 months (95%CI, 14.4–25.5), and 21.4 months (95%CI, 0–42.6) for stages IIIA, IIIB, IIIC, IIID, and IV, respectively.
Statistically significant differences have been reported in favor of those patients who completed adjuvant therapy, both in terms of RFS with a HR of 0.138 (0.040–0.471; p=0.002), and overall survival with an HR of 0.189 (0.057–0.624; p=0.006). No other statistically significant differences were observed based on the other variables, including the BRAF mutational status.
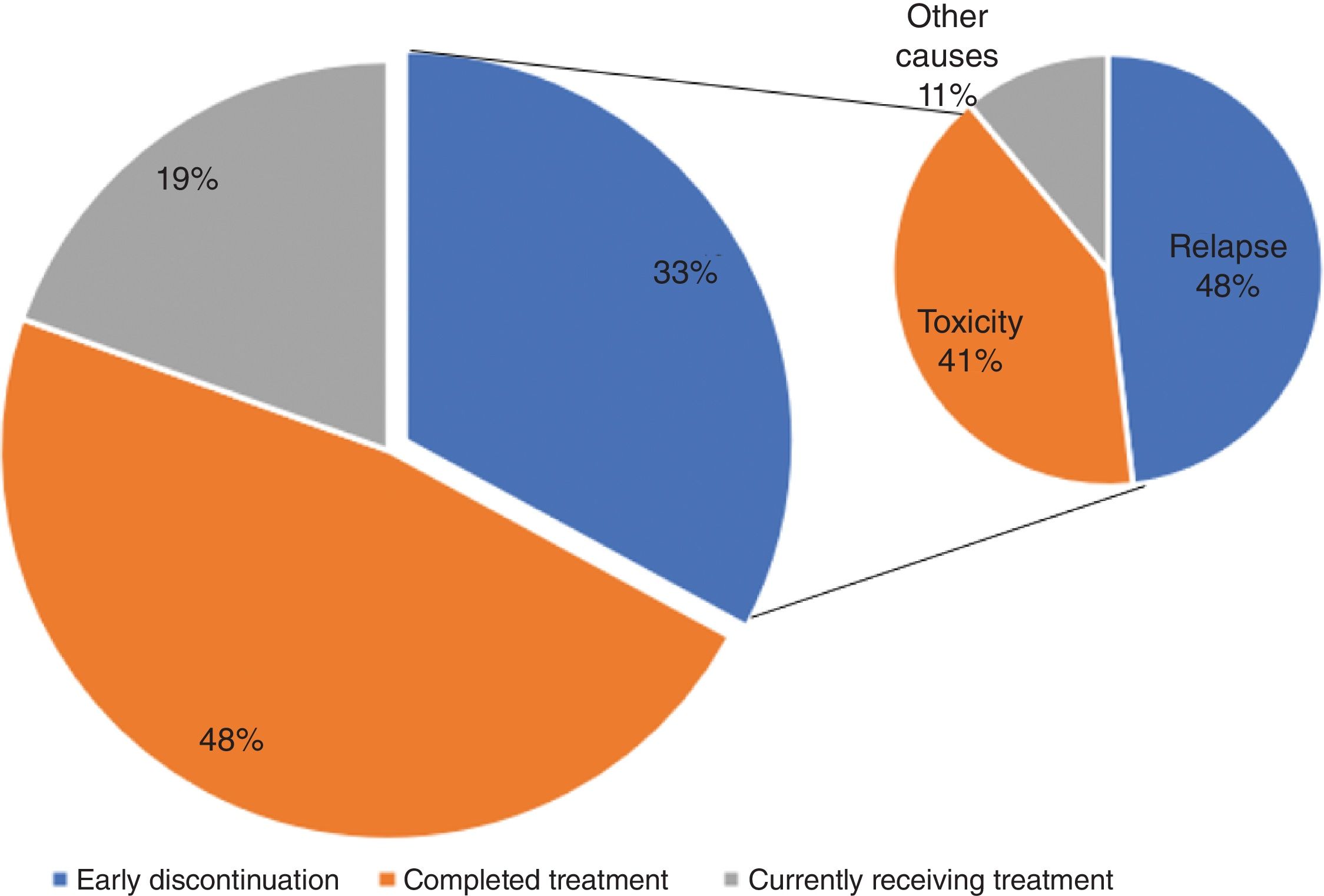
Causes for treatment discontinuation and recurrence patternsA total of 48% patients (n=39) completed the treatment. Of the patients who did not complete the treatment (n=41), 16 were still on it at the data cutoff, and 27 interrupted it prematurely due to relapse (n=13), toxicity (n=11), or other causes (n=3) (Fig. 1).
Up to 16 patients experienced disease recurrence (19.8%), most of them (n=13; 81.2%) during adjuvant therapy. Metastases occurred locally (n=8; 50%) and distantly (n=8; 50%).
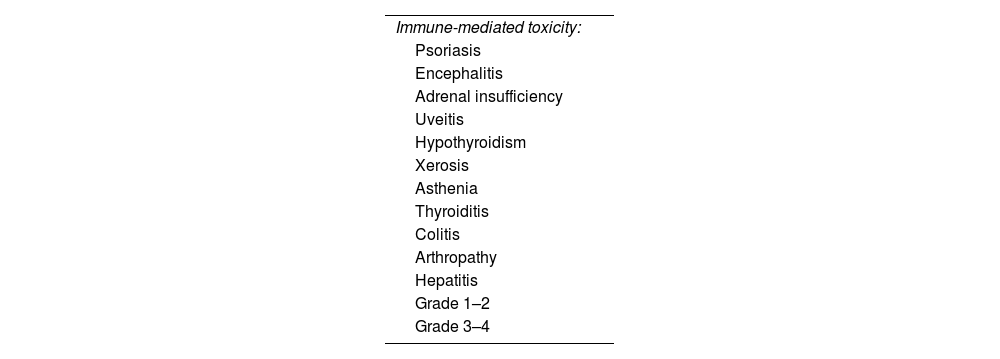
SafetyA total of 39.5% of patients experienced immune-mediated adverse effects (Table 2), most of which were mild, with a 34.5% toxicity rate being grade 1–2. A total of 12.3% of patients presented immune-related toxicity grade≥3. Permanent treatment discontinuation due to adverse effects occurred in 13.6% of cases (n=11).
The most common severe adverse effect was hepatic toxicity (n=4; 4.9%), followed by arthropathy (n=2; 2.4%), with isolated cases (n=1) of colitis, uveitis, encephalitis, and thyroiditis. No cases of pneumonitis or cardiac toxicity were recorded in our series.
There were 4 deaths during treatment in patients without recurrence, one of which was due to severe immune-mediated toxicity (thyrotoxic crisis and encephalitis).
DiscussionThe results of the CADIM study have been partially presented at various meetings and conferences.18 In this document, we present the final results of the 81 patients treated at the HCUVA and CHC. We consider the selected population to be representative of the overall population in our autonomous community, as these centers treat more than 90% of the total population of melanoma patients receiving adjuvant immunotherapy.
Regarding the analyzed population, the characteristics of our cohort are similar to those reported in other clinical practice trials, both in terms of sex,12,13,15,16 and age.12,13,16,19,20 In this series, the presence of melanoma of unknown primary origin is somewhat less common (3.7%) vs other clinical practice trials, where it accounts for 5.9% up to 11.9% of patients.12,13,16,19 The distribution by stage is similar to that of other studies; notably, the proportion of patients with resected stage IV melanoma (18.5%) is higher vs similar studies,12–15,19,20 except for the Italian expanded access analysis16 (23.1%). Also noteworthy is the percentage of patients on adjuvant therapy with mucosal melanoma (12.3%), which is also higher vs other studies (0.3% up to 2.8%).12,16 In our patients, most patients do not have a BRAF mutation, which is consistent with the distribution in most clinical practice series.12,16,19,20 The high proportion (30.9%) of patients with unknown BRAF status may be explained by the fact that this determination is not requested in the adjuvant setting, as it is not a determining factor for treatment decisions in this context, given that targeted therapy for this population is not funded in Spain. Lastly, regarding patient characteristics, a significant proportion of patients (79%) in the presented series underwent SLNB, which is similar to published series in the routine clinical practice (60% up to 65.8%)14,15,17; a similar situation occurs with the percentage of patients treated with lymphadenectomy: 45.7% in our series vs others (45.1% up to 85.8%).14,15,17,19
The results of RFS are numerically higher (84% at 1 year) vs those reported in clinical practice. The Austrian,14 Dutch,12 and Italian cohorts revealed RFS rates of 64.8%, 70.6%, and 76.6%, respectively. All of these reflect survival differences in favor of our study, possibly due to the limited number of patients, as the distribution by sex, age, and stage is similar, although the proportion of patients with mucosal melanoma, ulcerated melanomas, or resected stage IV in our series is higher, which does not initially favor greater survival.
In our study, 49.2% of patients completed treatment, 19.8% continued it, and 30.9% had discontinued treatment, most due to progression (n=13; 16%) and toxicity (n=11; 13.7%). Other analyses show wide variation in patients who complete treatment, ranging from 63.2%16 up to 42%,19 who discontinued treatment early due to relapse (18.7% and 19%, respectively) or toxicity (10.3% and 32%, respectively), among other causes.
Most patients who experienced disease relapse (n=13/16; 81.3%) did so during adjuvant therapy, a slightly higher rate vs the 76% observed by Owen et al.21
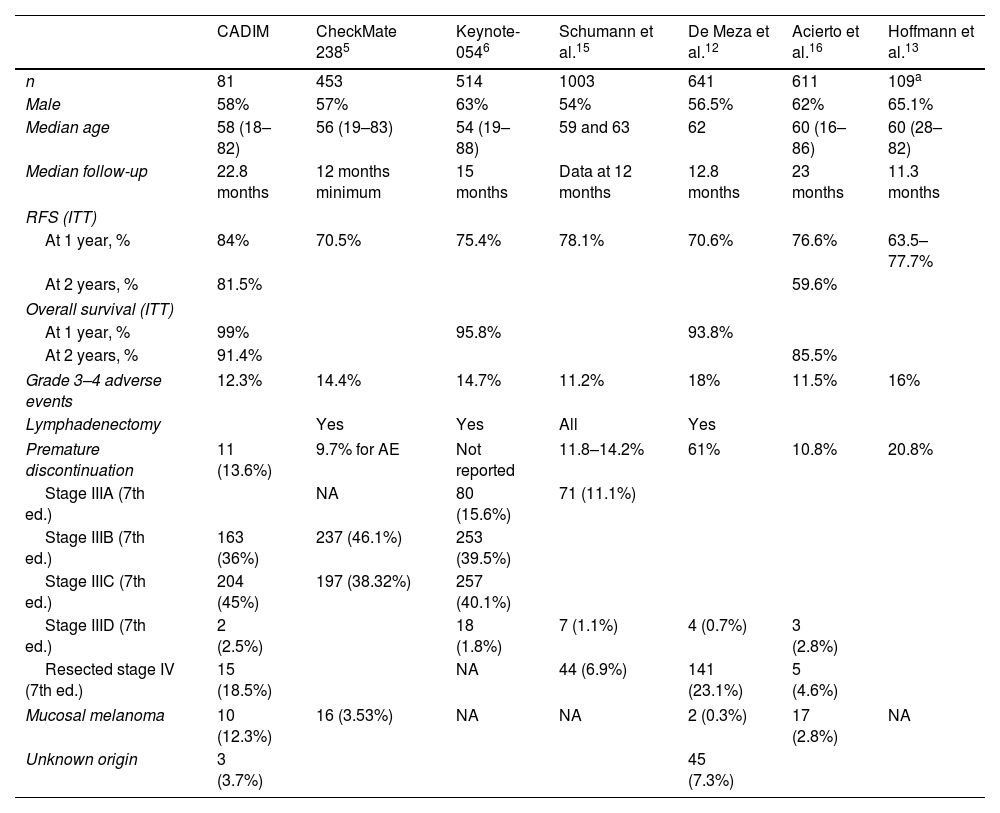
The different survival rates observed in the main clinical practice trials are shown in Table 3. Those studies with longer follow-up16,19,20 are the ones that also present higher RFS rates at 12 months, closer to our data (84% of patients free of relapse at 1 year). In our study, statistically significant differences were identified favoring patients who completed adjuvant therapy in terms of RFS and overall survival, although other studies19 do not find survival differences between those who completed treatment and those who discontinued early.
Comparative data of our population with clinical trials and real-world studies.
| CADIM | CheckMate 2385 | Keynote-0546 | Schumann et al.15 | De Meza et al.12 | Acierto et al.16 | Hoffmann et al.13 | |
|---|---|---|---|---|---|---|---|
| n | 81 | 453 | 514 | 1003 | 641 | 611 | 109a |
| Male | 58% | 57% | 63% | 54% | 56.5% | 62% | 65.1% |
| Median age | 58 (18–82) | 56 (19–83) | 54 (19–88) | 59 and 63 | 62 | 60 (16–86) | 60 (28–82) |
| Median follow-up | 22.8 months | 12 months minimum | 15 months | Data at 12 months | 12.8 months | 23 months | 11.3 months |
| RFS (ITT) | |||||||
| At 1 year, % | 84% | 70.5% | 75.4% | 78.1% | 70.6% | 76.6% | 63.5–77.7% |
| At 2 years, % | 81.5% | 59.6% | |||||
| Overall survival (ITT) | |||||||
| At 1 year, % | 99% | 95.8% | 93.8% | ||||
| At 2 years, % | 91.4% | 85.5% | |||||
| Grade 3–4 adverse events | 12.3% | 14.4% | 14.7% | 11.2% | 18% | 11.5% | 16% |
| Lymphadenectomy | Yes | Yes | All | Yes | |||
| Premature discontinuation | 11 (13.6%) | 9.7% for AE | Not reported | 11.8–14.2% | 61% | 10.8% | 20.8% |
| Stage IIIA (7th ed.) | NA | 80 (15.6%) | 71 (11.1%) | ||||
| Stage IIIB (7th ed.) | 163 (36%) | 237 (46.1%) | 253 (39.5%) | ||||
| Stage IIIC (7th ed.) | 204 (45%) | 197 (38.32%) | 257 (40.1%) | ||||
| Stage IIID (7th ed.) | 2 (2.5%) | 18 (1.8%) | 7 (1.1%) | 4 (0.7%) | 3 (2.8%) | ||
| Resected stage IV (7th ed.) | 15 (18.5%) | NA | 44 (6.9%) | 141 (23.1%) | 5 (4.6%) | ||
| Mucosal melanoma | 10 (12.3%) | 16 (3.53%) | NA | NA | 2 (0.3%) | 17 (2.8%) | NA |
| Unknown origin | 3 (3.7%) | 45 (7.3%) | |||||
The frequency of severe immune-mediated toxicities in our study was 12.3%, which is similar to the adjuvant studies, with 14.4% in the Checkmate 238 study8 and 14.7% in the Keynote 054, and to other clinical practice data,15,16 but lower than that reported in other studies,12,20 likely due to less stringent toxicity collection in retrospective studies such as ours.
Treatment discontinuation due to toxicity was similar that reported by Keynote 054 study (13.6% vs 13%) and lower than in other clinical practice studies with discontinuation rates ranging from 17.9% up to 22%.12,14
Despite the differences reported with other trials and studies and the limitations of indirect comparisons, we believe that the prolonged median follow-up in our study and the consistency in safety data validate the results obtained, supporting the clinical activity of immunotherapy in patients with resected stage III and IV melanoma.
As weaknesses, we should mention that our study is retrospective, and the number of patients is limited. In any case, most patients completed treatment, the follow-up of patients is prolonged, and we could obtain most of the considered variables.
ConclusionsProlonged follow-up will allow us to assess the possible development of long-term immune-mediated effects, characterize relapses in these patients, and determine the potential impact of early treatment discontinuation on survival. European initiatives, such as EuMelaReg22 will facilitate and standardize data collection, providing higher quality to clinical practice studies.
FundingNone declared.
Conflicts of interestNone declared.