Pruritus is the most common symptom of dermatologic and systemic diseases. The diagnosis of pruritus is clinical, although additional tests may be necessary to identify or confirm the cause. Translational medicine has led to the discovery of new mediators of itch, or pruritogens, as well as new receptors. Knowing how to properly recognize the main pathway that mediates itch in each patient is the key to successful treatment. Although the histaminergic pathway predominates in conditions like urticaria or drug-induced pruritus, it is the nonhistaminergic pathway that predominates in nearly all other skin diseases covered in this review. Part 1 of this 2-part review discusses the classification of pruritus, additional testing, the pathophysiology of itch and the pruritogens implicated (including cytokines and other molecules), and central sensitization to itch.
El prurito es el síntoma más frecuente asociado a enfermedades dermatológicas y sistémicas. Su diagnóstico es clínico, aunque en ocasiones será necesario realizar pruebas complementarias para identificar o confirmar el origen. La medicina traslacional ha permitido descubrir nuevos mediadores pruritógenos y nuevos receptores. Saber reconocer adecuadamente la principal vía por la que media el prurito en cada paciente será clave para el éxito terapéutico. La vía histaminérgica predomina en enfermedades como la urticaria o las reacciones a fármacos, mientras que la vía no histaminérgica predomina en prácticamente la mayoría de las otras dermatosis incluidas en esta revisión. La clasificación del prurito, las pruebas complementarias, la fisiopatología y los pruritógenos implicados, incluyendo citoquinas y otras moléculas, así como la sensibilización central al prurito que sufren estos pacientes formará parte de este primer manuscrito sobre el prurito.
Samuel Hafemreffer described pruritus in the seventeenth century in the same way we recognize this symptom today, that is, an unpleasant and uncomfortable somatic sensation for the patient,1 with a substantial sensorial, emotive, and motivational component causing an irresistible desire to itch. Translational medicine has enabled the identification of new participants in its complex pathophysiology.2 An interaction between skin, the immune system, and the nervous system triggers and sustains pruritus in the long term. As the poet Ogden Nash said, ‘Happiness is scratching yourself every time you feel an itch.’ However, patients with chronic pruritus experience a substantial negative impact on their quality of life.
EpidemiologyStänder et al.3 reported that 22–26% of the general population suffers from chronic pruritus. Leader et al.4 reported a figure of between 8% and 28% of the general population as suffering from this condition. Specifically, pruritus is present in 78% of patients aged over 65 years,5 87% of atopic patients,6 84% of patients with psoriasis,7 35% of patients in hemodialysis,8 and 45% of patients with HIV infection.9 The cause of pruritus is never determined in up to 44.5% of patients (pruritus of unknown origin).10
Classification of pruritusPruritus is considered chronic when it lasts for more than 6 weeks. The different types of pruritus are shown in Table 1.
Different types of pruritus.
| Neurogenic: Induced by pruritogenic and inflammatory mediators (peptidogenic and nonpeptidogenic), in absence of neuronal damage. Th2 lymphocytes appear to play a role in the development and maintenance of pruritus. |
| Neuropathic: This type accounts for 8% of all cases of chronic pruritus, where direct neuronal damage affects both integration and transmission of peripheral and central pruriception. Many of these patients experience pain. Causes include postherpetic neuralgia, entrapment syndromes (notalgia paresthetica, brachioradial pruritus, meralgia paresthetica), masses in the CNS, nerve fiber degeneration (diabetes mellitus, alcohol, chemotherapy), Fabry disease, and small-fiber neuropathy. This type of pruritus is usually associated with decreased nerve fiber density in the epidermis. |
| Pruriceptive: Activation of afferent sensory fibers by pruritogens in the skin. This can be triggered by external irritants or inflammatory skin diseases. |
| Psychogenic: There is a possible psychosomatic or psychiatric origin. Phobias, obsessive-compulsive disorder, psychotic diseases (ICD-10 F54 includes psychogenic pruritus among other somatoform disorders). Psychiatric pruritus has a prevalence of 32% among patients with the disease, where the scalp is the most frequently involved site. |
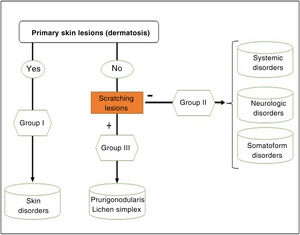
We can classify pruritus according to whether the origin is dermatologic, systemic, neurologic, psychogenic, of mixed origin, or others (this latter category includes idiopathic). However, the classification recommended by the International Forum for the Study of Itch (IFSI)11 has 3 groups:
- -
Group I or primary: chronic pruritus on altered or inflamed skin (primarily).
- -
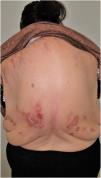
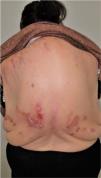
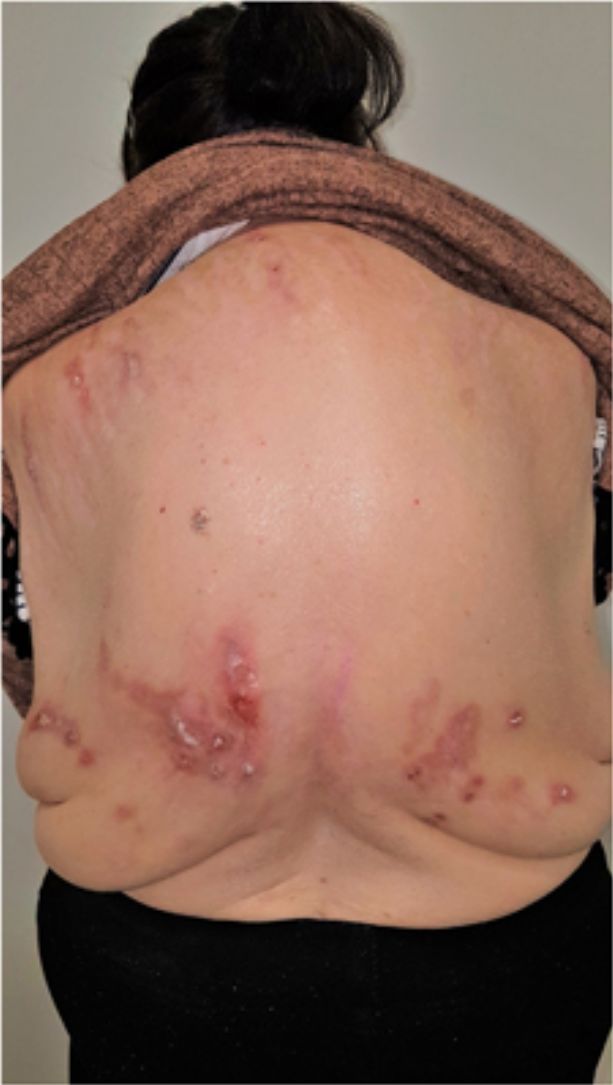
Group II or somatoform (previously denoted pruritus sine materia): chronic pruritus in an area of skin previously not involved. Pruritus in this group is also sometimes denoted as invisible dermatosis. An example is dermatitis factitia. A classic sign during the examination is the butterfly sign (Fig. 1).
- -
Group III or predominance of excoriation: chronic pruritus with itching lesions.
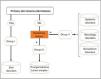
The algorithm in Fig. 2 can be followed to identify the group in which each patient belongs.
Table 2 shows some of the diseases most frequently associated with pruritus.
Systemic diseases associated with pruritus.
| Metabolic/endocrine disorders | Hyperparathyroidism, iron deficiency, diabetes mellitus, alcoholism, hyper- and hypothyroidism, carcinoid syndrome |
| Hepatic/biliary | Primary biliary cholangitis, primary biliary cirrhosis, infectious hepatitis |
| Renal | Chronic kidney failure |
| Autoimmune disorders | Dermatomyositis, systemic sclerosis, Sjögren syndrome |
| Infectious | HIV, helminth and parasitic infection, viral hepatitis (HVB, HVC), scabies, dermatophytosis, onchocerciasis, varicela |
| Hematologic | Polycythemia vera, essential thrombosis, Hodgkin and non-Hodgkin lymphoma, ferropenic anemia, mastocytosis, multiple myeloma |
| Neurologic | Multiple sclerosis, brain tumors, notalgia paresthetica, brachioradial pruritis, postherpetic neuralgia, small fiber neuropathy |
| Psychiatric | Depression, anxiety, delusional disorders, anorexia nervosa |
Premonitory pruritus is when the condition appears months or even years before a systemic or neoplastic disease. Studies have been published of the risk of hematological neoplasms12 or bile duct neoplasms in patients previously diagnosed with pruritus.
Medical historyTable 3 shows the important details in the medical history of patients with pruritus, both in general terms and for specific orientation according to its origin.
Medical history of patients with pruritis.
| Questions focused on pruritus | 1. Date of onset and duration2. Site (appearance, propagation)3. Type of pruritus (inflammatory vs. neuropathic)4. Intensity or severity5. Course: diurnal fluctuations, continuous course/without touch, spontaneous improvement/worsening6. Trigger factors; relief factors7. Scratching behavior8. Temporal association with pre-existing diseases, surgery, medication intake, other events.9. Prior treatments (with/without resolution)10. Patient's own opinion about the cause11. Factors of psychosocial burden: deterioration in health-related quality of life, mental anguish, sleep disorders |
| General questions | 1. Pre-existing diseases (including dermatoses)2. Medication intake, blood transfusion3. Prior surgical interventions4. Allergies: Type I and type IV allergies5. History of atopy6. Travel history7. Pregnancy |
| Directed questions | 1. Family members affected by nocturnal pruritus→scabies2. Pruritus after contact with water→aquagenic pruritus, rule out polycythemia vera3. Exercise-induced pruritus→cholinergic pruritus4. B symptoms→lymphomas, neoplasms5. Jaundice→Involvement of the biliary tract/liver6. Worsening in winter→asteatotic eczema, xerosis |
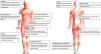
Fig. 3 shows the main diagnoses according to site.
Additional testsDiagnosis of pruritus is based on clinical observation. Patients with pruritus classified as group II and III are candidates for additional tests, depending on the medical history and suspected diagnosis. Patients with skin lesions may need microbiological cultures, skin biopsy (with or without immunofluorescence detection), indirect immunofluorescence, 24-h urine test for porphyrias, and study of fresh samples for mycosis and scabies. The general blood workup should include creatinine, urea, estimated glomerular filtration rate, potassium, glucose, ferritin, bilirubin, transaminases (GOT, GPT, GGT), alkaline phosphatase, lactate dehydrogenase, thyroid stimulating hormone, complete hemogram, urinalysis (reactive strip), globular sedimentation rate (GSR), and C reactive protein (CRP). Study of fasting bile acids levels in blood should be requested for pregnant patients if there is suspicion of cholestasis.
Targeted additional testsTable 4 shows the specific additional tests for patients with pruritus.13
Additional targeted tests in patients with pruritus.
| Anal pruritus | Study of parasites, tapeworm eggs, digital rectal examination, PSA |
| Aquagenic and genital pruritus, pruritus of unknown origin | Lactose/sorbitol intolerance test |
| Hemogram abnormalities/suspicion of lymphoproliferative diseases | Vitamin B12, folic acid, protein electrophoresis, immunofixation, JAK2 status, bone marrow biopsy (if necessary) with (immuno)cytology and histology |
| In the case of iron deficiency/fecal irregularities | Guaiacol test in feces |
| In the case of suspicion of hepatobiliary disease | Hepatitis serology (anti-HAV, HbsAg, anti-HBc, anti-HCV), bile acids, antimitochondrial antibodies (AMA), perinuclear antineutrophil cytoplasmic antibodies (pANCA), antinuclear antibodies (ANA), smooth muscle antibodies (SMA), soluble liver antigen antibodies (SLA), liver-kidney microsomal (LKM) antibodies, tissue transglutaminase antibodies, alpha-fetoprotein (in the case of liver cirrhosis/hepatic mass) |
| In the case of pathologic fasting glucose levels | HbA1c, glucose tolerance test |
| In the case of suspicion of endocrine disorders | Parathyroid hormone, phosphate, calcium, T3, T4, 25-OH cholecalciferol, TSH anti-receptor antibodies (TRAH), TSH receptor antibodies (TRAb), thyroid peroxidase antibodies (TPO-Ab) |
| In the case of suspicion of HIV | HIV serology, syphilis serology (if necessary) |
| In the case of suspicion of mastocytosis | Tryptase levels, methylimidazole acetic acid in 24-h urine |
| In the case of suspicion of neuroendocrine tumors | Chromogranin A, 5-hydroxyindolacetic acid in 24-h urine |
| In the case of suspicion of allergy | Total IgE, specific IgE (if necessary), prick test, skin patch tests |
Imaging tests are requested in accordance with the initial clinical suspicion. Chest X-ray is considered standard, but abdominal ultrasound, computed tomography, magnetic resonance imaging, gastroscopy, ultrasound-guided lymph node biopsy, etc. can also accompany the laboratory work-up as per the clinician's judgment. Management of patients with pruritus requires multidisciplinary teams and collaboration across specialties.
Scales for measuring pruritusIn clinical practice, the most widely used scales are the numerical evaluation scale (NES) and the visual analog scale (VAS). A scale validated in Spanish is available, under license, the ItchyQoL,14 a 22-item questionnaire that measures symptoms, functions, emotions, and self-perception. Comparing outcomes in improvement in pruritus in clinical trials is a complex task with certain limitations because there is no standard measurement of pruritus severity, and different measurement instruments are used.
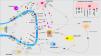
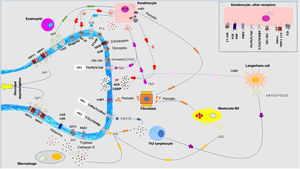
Pathophysiology of pruritus: interaction between neurologic, immune, and cutaneous systemsIn recent years, the close relationship between the skin, immune system, and nervous system has been extensively investigated. The participation of each of these systems plays a fundamental role, both in the development of pruritus and in sustaining the condition in the long term. Although each system is presented separately, in truth, they do not exist in isolation but rather as a whole. Fig. 4 shows a schematic and summarized representation of the main pruritogenic mediators and receptors.
A multitude of receptors are expressed in the nerve endings of cutaneous C fibers. Depolarization is triggered by calcium ion entry through TRPA1 and TRPV1. These are activated by binding to the receptor of the corresponding molecule. The different pruritogens are released by keratinocytes, mastocytes, Th2 lymphocytes, macrophages, and fibroblasts. They form positive feedback loops with different pruritogens such as periostin and TSLP. Release by nerve endings of substance P (SP) and calcitonin-gene related peptide (CGRP) act on keratinocytes and Langerhans cells, respectively, potentiating the neuroinflammatory environment. Abbreviations: 5HT, serotonin; ACh, acetylcholine; CB1-CB2, cannabinoid receptor; ET-1, endotelin-1; ET-1R, ET-1 receptor; H1R-H4R, histamine receptor; KOR, kappa opioid receptor; MOR, mu opioid receptor; NGF, neuron growth factor; NK-1R, neurokinnin-1 receptor; ST2, IL-1-like receptor; TrkA, tropomyosin kinase A receptor.
The afferent sensory nerves, which are heterogenous and polymodal, can be classed as Aδ fibers (measuring 2–5μm in diameter, myelinated, and with a rate of conduction of 8–15m/s) or C fibers (measuring 0.2–1.5μm in diameter, unmyelinated, with a rate of conduction of 1–2m/s). The nerve endings form part of the skin microenvironment along with the microbiome, keratinocytes, and immune system cells, interacting with them via both receptors of peptide mediators (substance P [SP] and calcitonin-gene related peptide [CGRP]) and receptors of non-peptide ones (artemin, neurotrophin). Pruriceptive C fibers make up at least 10% of all the sensitive neuronal population in the skin.15 Four classes of nonpeptide neurons have been described16: NP1 (neurons positive for the MrgprD-associated pruritis receptor), NP2 (neurons positive for histamine-1 receptor, MrgprA3, and mrgprC11), NP3 (neurons positive for histamine-1 receptor, natriuretic polypeptide b receptor, IL31 receptor, and oncostatin M receptor, as well as JAK1), and NP4.
Unmyelinated C fibers are low-threshold mechanoreceptors triggered by pain, thermal stimuli, and pruritogens. They express receptors for proteases, cytokines, and voltage-gated ion channels (TRPA1 and TRPV1). These C and Aδ fibers (first-order neurons) are projected directly toward the spinal cord passing through the dorsal root ganglion, forming synapses with the interneurons and second-order neurons in the posterior horn of the spinal gray matter (mainly lamina I and II).
Pruritogenic mediatorsHistamineThis main histaminergic mediator of unmyelinated C fibers is secreted by mastocytes, basophils, neurons, and keratinocytes.17 Both TRPV1 and TRPA1 are implicated in central histamine signaling after activation of their receptor.
Substance PThis neuropeptide of the tachykinin family is implicated in nonhistaminergic pruritus.18 It is produced and secreted by nerve fibers and induces mastocyte degranulation and secretion of neuronal growth factor (NGF) by keratinocytes. Its receptors, neurokinin-1 (NK-1R) and Mrgpr, are expressed in C fibers, keratinocytes, endothelial cells, and immune system cells. Patients with chronic pruritus show overexpression of SP.
SerotoninThis neurotransmitter is released by mastocytes, platelets, and endothelial cells, and is able to activate C and Aβ fibers.19 It has been associated with uremic and cholestatic pruritus.
Endotelin-1This potent peptidergic vasoconstrictor is secreted by keratinocytes that participate in mediation of pruritus through its A receptor.20 Its secretion is induced by activation of PAR2, among others.
Neuropeptide Y (NPY)Spinal inhibitory interneurons express receptors for this molecule which form synapses with afferent fibers excited by secretion of natriuretic peptide b.21
Natriuretic polypeptide b (nppb)Secreted by the central nervous system, this molecule stimulates release of pruritogens by keratinocytes and dendritic cells.22 It activates TRPV1, MrgprA3, and MrgprC11 in peripheral afferent fibers. Its central signaling occurs through disinhibition of GRPR+ excitatory interneurons. It has been proposed as a key molecule in the development of pruritus in patients with atopic dermatitis.
PeriostinThis protein is secreted by fibroblasts that induce pruritus dependent on binding to integrin αvβ3 directly at the pruriceptive nerve endings, as well as activation of TRPA1 and TRPV1.23 Feedback from TSLP and nppb affects its activity. It enhances Th2-lymphocyte-mediated inflammatory response and participates in the process of keloid fibrosis and systemic sclerosis.
TRP ion channels (TRPV1-TRPA1-TRPV4-TRPM8)Receptors of the vanilloids family are expressed in peptidergic neurons that respond to bradykinin, prostaglandins, and retinoids.24 TRPV1 (ankyrin family) is activated by mustard, garlic, and tetrahydrocannabinol (THC). TRPV1-mediated neuronal depolarization induces release of peptidergic mediators (SP and GRPR). TPRV4, expressed in keratinocytes, neurons, and macrophages, participates as a mediator for thermal, chemical, osmotic, and mechanical stimuli as well as in serotonin-induced pruritus. TRPM8 is a cold sensor activated by menthol.
ReceptorsNeurokinin-1 receptor (NK-1R)This receptor belongs to the family of G-protein coupled receptors.25,26 The complete isoform shows high affinity for SP. It is expressed in nerve fibers, keratinocytes, mastocytes, and fibroblasts. This receptor is expressed in the peripheral and central nervous system. Between 89% and 94% of neurons of the ascendent pathway of the spinothalamic and spinoparabrachial tract express it. In the brain, it is expressed in the thalamus, cingulate cortex, basal ganglion, and cerebellum. Overexpression of NK-1R has been demonstrated in the epidermis of skin affected by pruritus. The antagonists of this receptor can be used as a target in the treatment of pruritus.27
G-protein coupled receptorsThese 7-transmembrane receptors are expressed both in nerve fibers and mastocytes. The receptors mediate nonhistaminergic pruritus at the peripheral level.28 There is substantial neuroimmune interaction between mastocytes and the nerve fibers given their proximity in biopsies of urticaria or drug-induced reactions. In 2001, the Mas-related G protein-coupled receptors (Mrgpr), implicated in acute and chronic pruritus, were discovered. SP binds to MrgprX2 expressed in mastocytes, inducing IgE-independent degranulation. In cholestatic pruritus, bile acids show affinity for MrgprX4.29 MrgprX2 is the focus of investigation in inflammatory dermatoses that are accompanied by pruritus.30
Opioid receptorsThese receptors form part of a group of G-protein coupled receptors. Endogenous endorphins show affinity for μ opioid receptors (MOR) expressed in C and Aβ fibers. These form synapses with GRPR+ excitatory interneurons in the posterior horn, where signaling is pruritogenic. The K opioid receptors (KOR) bind to dynorphin, whose activity is antipruritogenic.31 There is a physiological equilibrium between MOR and KOR activation, controlled mainly by excretion of dynorphin. At the level of the spinal cord, there is a subpopulation of dynorphin+ inhibitory interneurons. Loss of function of these interneurons is implicated in central signaling of pruritus and alloknesis. Butorphanol (KOR agonist/MOR antagonist) decreases brain activity associated with itching.32 A reduction in IL31 levels has been demonstrated in patients treated with opioid derivatives.33
Histamine receptors (HR)These are G-protein coupled receptors that indirectly activate TRPV1 through lipoxygenases. HR-4 is present in keratinocytes and in C fibers.34 Although most antihistamine agents act as selective antagonists of H1R, it has also been shown that cetirizine and rupatadine inhibit the action of PAF,35 cetirizine decreases secretion of IL4,36 fexofenadine inhibits release of triptase,37 and ebastine inhibits the Th2-mediated inflammatory pathway.38
Protease-activated receptors (PAR)These receptors are expressed in neurons, immune cells, astrocytes, and keratinocytes.39 Activation of these receptors triggers transcription of cytokines, chemokines, and growth factors.
Endothelin-1 receptor AThis receptor is a nonhistaminergic pruritogenic mediator, whose signaling occurs via neural peptide endothelin converting enzyme 1 and the Erk1/2 pathway.20 This pathway is overexpressed in prurigo nodularis.
OthersThe toll-like receptors (TLR),20 lysophosphatidic acid 5 receptor,40 and the leukotriene cysteinyl 2 or 4 receptor (LTC4)41 have also been implicated in mediation of pruritus.
CytokinesIL31/OSMt42IL31 was discovered in 2004. It is produced and released by CD4+ T lymphocytes, mastocytes, macrophages, basophils, eosinophils, keratinocytes, and dendritic cells. Its heterodimeric receptor (IL31 receptor [IL31R]/oncostatin M receptor β subunit [OSMRβ]) is expressed in the skin and brain, among other organs. IL31 is coexpressed with TRPV1 and TRPA1, implicated directly in pruritus. Th2 lymphocytes release this cytokine under different stimuli, as do mastocytes through activation of Mrgpr. Pathological secretion of IL31 leads to impaired skin barrier function, both due to its influence on differentiation and expression of filaggrin and to proliferation and differentiation of keratinocytes (acanthosis, parakeratosis, and epidermal hyperplasia).43 Intracellular IL31 signaling occurs via the JAK1/STAT3, Ras-Erk1/2, and PI3K/AKT pathway. Another neuroimmune role played by IL31 is that of neuropoietic simulation leading to an increase in skin nerve fibers.44 Elevated levels have been detected in atopic dermatitis45 and prurigo nodularis,46 with a certain degree of correlation with disease severity.
IL4/IL13These landmark cytokines of the type 2 inflammatory pathway are produced and released mainly by Th2 lymphocytes, basophils, and mastocytes. Both act directly on nerve endings that express their receptors.47 The heterodimeric receptor IL4Rα/IL13Rα1, when activated, induces initial JAK phosphorylation to trigger intracellular signaling (in this case, activation of the voltage-gated ion channels for neuronal depolarization). IL4 is able to enhance neuronal response to other pruritogens such as chloroquine, histamine, IL31,48 and TSLP.
Alarmins (IL33, thymic stromal lymphopoietin [TSLP])These molecules, secreted by keratinocytes, induce type 2 immune polarization through OX40L/OX40 signaling.49 The TSLP receptor is expressed in sensory neurons,50 whereas the IL33 receptor, ST2, is a heterodimer shared with IL1,51 which transmits its signal via TRPV1 and TRPA1. In humans, blocking these cytokines with etokimab (anti-IL33) or tezepelumab (antiTSLP) has not led to significant improvement in atopic dermatitis.
Janus kinase (JAK)JAKs are proteins of a family of enzymes associated with cytokine receptors.52,53 Currently, 4 subtypes have been reported: JAK1, 2, and 3, and TYK2. These receptors autophosphorylate via the ATP molecule to activate the STAT3/STAT6 pathway. IL31, IL4, and IL13 transmit signals via JAK1, and participate in the development of neuroinflammatory pruritus. JAK1 inhibitors have demonstrated rapid and effective control of pruritus in atopic dermatitis. The development of topical JAK inhibitors such as delgocitinib and ruxolitinib has opened a window of opportunity for local control of pruritus.
CellsKeratinocytesThese are considered on the frontline of the nervous system given their high bioactivity and ability to secrete pruritogens.54 They express PAR2, TRL3, HR1-HR4, endothelin A and B, 5-HTR, OSMRβ, integrin αvβ3, TSLPR, NPY receptors, including TRPV3, and V4. Impairment of the epidermal barrier function by different noxae may be exacerbated by secretion of pruritogens, which increase the inflammatory gradient and transepidermal water loss.
Merkel cellsThese cells participate in triggering the itch-scratch cycle triggered by mechanical stimuli through the Piezo2 receptor.55 The complex formed by these cells and the MrgprA3+ pruriceptive endings (C fibers) in cases of dry skin and prurigo nodularis may be functionally impaired. They express TRPM8.
MastocytesThese cells are loaded with pruritogens, able to activate the histaminergic and nonhistaminergic pathway.56 They are localized close to nerve fibers that participate in potentiating neuroinflammatory pruritus.
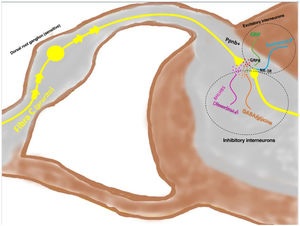
Signal transmission along the spinal cord (ascending pathway)Peripheral axons are projected directly toward the posterior horn of the spinal cord (exterior region of lamina I and II for peptidergic pathways, and the interior region of lamina II for nonpeptidergic pathways), after passing through the dorsal root ganglion. Between 2% and 5% of the spinal neuronal population participates in transduction of pruritus, where NK-1R is the main receptor. The presence of excitatory interneurons (SP+ and GRPR+) and inhibitory ones (GABA+) plays a key role in modulating ascending signaling in pruritus. These interneurons express VGLUT2 (vesicular glutamate transporter), neurotensin, and somatostatin. The presence of BHLH+ (basic helix loop helix) interneurons is very important given that they regulate neurogenesis and define neuronal identity within the spinal cord. The BHLHB5+ interneurons are of the inhibitory type,57 and can be activated by menthol, capsaicin, or dynorphin. They express somatostatin and NPY receptors. Another population of inhibitory interneurons are the GABAergic ones, localized in lamina I and III. The neurons also coexpress the glycine receptor,58 another inhibitory neurotransmitter. When a clear excitatory polarization of excitatory GRPR+ interneurons is present, recruitment of enkephalinergic interneurons is induced to mitigate the intense stimulus for itching. Another subpopulation of excitatory interneurons expresses SOM+. This expression enhances pruritus signaling to second-order neurons. Activation of BHLHB5+ with dynorphin decreases the sensation of pruritus. Signaling via the pruriceptive fibers that reach the posterior horn induces secretion of nppb, which activates excitatory interneurons of gastrin secretor peptide. Fig. 5 shows a schematic view of the above.
The depolarized C fibers that transmit the pruriceptive signal release nppb when they reach lamina I and II of the posterior horn of the spinal cord, stimulating the excitatory interneurons that express GRP. Another of the excitatory interneurons expresses the NK-1 receptor for substance P. There are inhibitory interneurons, BhbIB5 is one of these. These are important in that they release dynorphin A, and also GABA and glycine, thereby impeding central signaling of pruritus. Loss of this inhibitory function is one of the causes of central sensitization in pruritus. Abbreviations: BHLHB5, B helix-loop-helix protein; GRP, gastrin-releasing peptide; GRPR, GRP receptor; NK-1R, neurokinin receptor 1; nppb, natriuretic polypeptide b.
Interneurons are implicated in the door control theory, the aim of which is to control the nociceptive and non-nociceptive signaling in the central nervous system. Loss of control could be responsible for central pruritus signaling,59 present in patients with chronic pruritus. Activation of inhibitory interneurons is crucial to prevent signaling from passing to second-order neurons projected into the central nervous system, where pruritus is processed and will give rise to the motor component (scratching), but also the emotive component (pleasure after scratching), necessary to sustain the pruritus-scratching cycle.60
Processing pruritus in the brain (CNS)Thanks to neurobiology, today, we know the areas of the brain implicated in the pruritus circuit. The first station that receives the fibers of the spinothalamic and trigeminothalamic tract is the thalamus (mainly the ventrobasal complex), and the parabrachial nucleus.61 The thalamus for its part contains neurons that can be activated by pruritogens, whether histaminergic or nonhistaminergic. More than 70% of the neuronal population of the posteromedial region of the ventrobasal area of the thalamus responds to more than one type of pruritogen. In the somatosensory cortex, the intensity and localization of the pruritus is processed, and the motor signal for scratching is emitted. The prefrontal cortex and amygdala mediate the emotive and pleasure component implicated in scratching.62 The amygdala63 is activated by pruritogenic stimuli and deactivated by scratching; it participates actively in the sensory and affective process of pruritus. GABAergic neurons have been detected in the periaqueductal gray matter and the ventral tegmental area. These neurons participate in the disagreeable and aversive perception of pruritus. They are activated with active pruritus in the patient, possibly playing an important role in the patient's addiction to scratching. There is a true motivational component in pruritus, namely, the urgent need for scratching. The anterior cingulate cortex and motor and premotor cortex participate in this process. In fact, these neurons are deactivated when the patient actively scratches.
Descending pathway of pruritus: motor action of scratchingThe main neurotransmitters are noradrenaline (NA) and serotonin (5-HT).64 The main population of NA+ neurons are localized in the locus coeruleus, whereas their adrenergic receptors α1 are present in the spinal cord inhibitory interneurons.65 The 5-HT1A receptor expressed in GRPR+ interneurons also participates directly in the descending signaling of pruritus. The periaqueductal gray matter (PAG) receives signals both from the amygdala and the parabrachial nucleus, actively participating in the processing of pruritus. A subpopulation was discovered of tachykinin-1 expressing glutaminergic neurons (TAC1+) that facilitated the itch-scratch cycle.66 Ablation of these neurons reduces scratching induced by pruritus, while activation generates the action of scratching. The anterior cingulate cortex forms a circuit with the anterolateral thalamic nucleus and the dorsomedial striatum, modulating pruritus of histaminergic origin through the spinal circuit dependent on BHLHB5 interneurons.67
Conflicts of interestThe author declare that he has no conflicts of interest.