Although Mercromina Film and other topical antiseptics are widely used, they are not included in the standard series recommended by the Spanish Contact Dermatitis and Skin Allergy Research Group for testing suspected allergic contact dermatitis (ACD). Furthermore, no recent studies have investigated the allergenic potential of merbromin.
ObjectiveTo determine the allergenic potential of merbromin and compare it with that of other topical antiseptics widely used in clinical practice, including povidone-iodine, chlorhexidine, and eosin.
Material and methodsProspective single-center observational safety study of 105 patients with suspected ACD seen at the dermatology department of our hospital.
ResultsOf the 105 patients studied, 1.9% had a positive patch test to merbromin and 12.4% were sensitized to povidone-iodine. The differences in the proportion of patients with ACD to Betadine Solución Dérmica (povidone-iodine) compared with the rest of the antiseptics was statistically significant (McNemar test, P<.05). No adverse reactions were observed in any of the patients.
ConclusionsBased on the patch tests conducted, Mercromina Film has very low allergenic potential. The highest allergenic potential was observed for povidone-iodine.
Pese al uso tan extendido de la Mercromina Film® y otros antisépticos de uso tópico, estos no se encuentran incluidos en la serie estándar española del Grupo Español de Investigación en Dermatitis de Contacto y Alergia Cutánea, realizada ante sospecha de dermatitis alérgica de contacto. Además, no existen estudios recientes sobre capacidad alergogénica de la merbromina, motivo por el que se plantea la presente investigación.
ObjetivoDeterminar la capacidad alergénica de la merbromina y compararla con la de los otros antisépticos de uso tópico frecuentemente utilizados en la práctica clínica, entre los que se incluye la povidona iodada, clorhexidina y eosina.
Material y métodosEstudio observacional de seguridad, prospectivo y unicéntrico realizado en 105 pacientes con sospecha de dermatitis alérgica de contacto que acudieron a la consulta del departamento de dermatología.
ResultadosEl 1,9% de los participantes presentó dermatitis alérgica de contacto a la merbromina. El 12,4% de los pacientes presentó sensibilización ante la povidona iodada. Las diferencias entre el porcentaje de pacientes que presentó dermatitis alérgica de contacto a Betadine® solución dérmica respecto al resto de antisépticos fueron estadísticamente significativas (prueba de McNemar; p<0,05). No se registraron efectos adversos con ninguno de los antisépticos en estudio.
ConclusionesMercromina Film ha demostrado una muy baja capacidad alergénica en la prueba de patch test. Cuando se comparan con otros antisépticos tópicos, la mayor capacidad alergénica se encontró con la povidona iodada.
Topical antibiotics are considered the treatment of choice in superficial skin infections and infected wounds although increased resistance of Staphylococcus aureus to topical antibiotics has been observed.
As bacterial resistance does not develop with antiseptics, it would be pertinent to extend our knowledge of the irritant/allergenic capacity of these agents. All antiseptics have an irritant capacity, particularly when applied to lesioned skin. Allergic contact dermatitis occurs less frequently and is often underdiagnosed.
Mercromina Film (Spanish tradename) is an antiseptic indicated for disinfecting small superficial wounds, cracks, burns, and grazes.1 It has been marketed in Spain since 1971 by Laboratorio Lainco SA and, according to the Anatomical Therapeutic Chemical classification, is considered a mercury product (D08AK).
Mercury derivatives can cause local and systemic allergic contact dermatitis (baboon syndrome). The standard Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC) series includes 2 mercury derivatives: thiomersal and mercury metal.
The prevalence of allergic contact dermatitis is 5% to thiomersal and 3.7% to mercury metal.2 Cross-reactions occur between organic and inorganic mercury derivatives and so it is recommended to avoid mercury-based antiseptics in patients sensitized to this metal. The use of mercury-based antiseptics can also cause sensitization.
In a study from 1991, merbromin only induced an allergenic response in 1% of tests.3 Despite this finding, recent reviews still do not recommend use of mercury-based antiseptics because of their allergenic capacity and recommend replacing them with other safer antiseptics.4
Given that there are no recent studies on the allergenic capacity of merbromin, the primary objective of the present study was to determine the percentage of patients who present allergic contact dermatitis to the most common topical antiseptics: merbromin, chlorhexidine, povidone-iodine, and eosin. As secondary objectives, we compared the percentage of patients who presented with allergic contact dermatitis to these antiseptics and investigated concomitant allergies produced by the antiseptics of the study.
Material and MethodsThis was a prospective, observational, comparative safety study in patients with clinical suspicion of allergic contact dermatitis. In total, we recruited 105 consecutive patients who attended the dermatology department of the Hospital Clinic in Barcelona, Spain, for skin patch tests between March and November 2014. The inclusion criteria for the study were adult age and suspicion of allergic contact dermatitis. All participants signed the informed consent form prior to inclusion. No patient had concurrent diseases of relevance for the study. All study procedures were performed according to the Declaration of Helsinki and approved by the ethics committee of the hospital.
Assessment of the Skin Patch TestThe standard GEIDAC battery, specific batteries selected according to the reason for the referral, and the topical antiseptics object of the study were applied to the study participants. Topical antiseptics included (Spanish tradename given): Mercromina Film (active substance: merbromin 2%), Mercromina Film without any active substance, Cristalmina (active substance: chlorhexidine digluconate 10mg/mL), Betadine dermal solution (active substance: povidone-iodine 10%), eosin 2% (magistral formula). All antiseptics were applied with occlusion for the first 48hours.
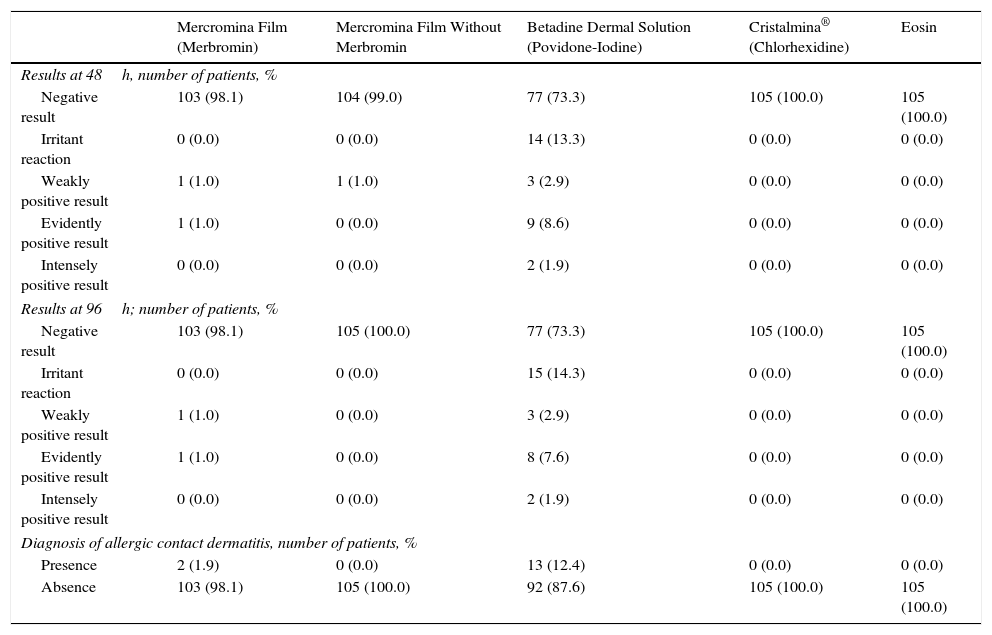
The tests were read at 48 and 96hours and the results were interpreted according to the parameters shown in Table 1. A patient was considered to have allergic contact dermatitis to an antiseptic when the result at 96hours was weakly positive, evidently positive, or intensely positive.
Sample Size Calculation and Statistical AnalysisAssuming that 1% of the patients had allergic contact dermatitis to merbromin,3 and assuming that 10% of the patients would not be analyzable, the sample size required to give a precision of±2.8% was 105. Categoric variables were described by absolute and relative frequencies (%), while the mean and SD were calculated for continuous variables. Comparisons of patients positive for antiseptics were made using the nonparametric Mann-Whitney U test. The McNemar test was used to compare the percentage of patients with allergic contact dermatitis obtained with one antiseptic compared with another. Statistical significance was set at P≤.05. All statistical procedures were performed using the SAS version 9.3 statistical package.
ResultsOf a total of 105 participants in the study, 84 were women (80.0%) and the mean age was 49.4±16.7 years.
The results showed that 12.4% of the patients in the study had allergic contact dermatitis to Betadine dermal solution and 1.9% to Mercromina Film (Table 2). No patient presented allergic contact dermatitis to Mercromina Film without active substance, Cristalmina, or eosin.
Results of Patch Tests and Final Diagnosis of Allergic Contact Dermatitis.
| Mercromina Film (Merbromin) | Mercromina Film Without Merbromin | Betadine Dermal Solution (Povidone-Iodine) | Cristalmina® (Chlorhexidine) | Eosin | |
|---|---|---|---|---|---|
| Results at 48h, number of patients, % | |||||
| Negative result | 103 (98.1) | 104 (99.0) | 77 (73.3) | 105 (100.0) | 105 (100.0) |
| Irritant reaction | 0 (0.0) | 0 (0.0) | 14 (13.3) | 0 (0.0) | 0 (0.0) |
| Weakly positive result | 1 (1.0) | 1 (1.0) | 3 (2.9) | 0 (0.0) | 0 (0.0) |
| Evidently positive result | 1 (1.0) | 0 (0.0) | 9 (8.6) | 0 (0.0) | 0 (0.0) |
| Intensely positive result | 0 (0.0) | 0 (0.0) | 2 (1.9) | 0 (0.0) | 0 (0.0) |
| Results at 96h; number of patients, % | |||||
| Negative result | 103 (98.1) | 105 (100.0) | 77 (73.3) | 105 (100.0) | 105 (100.0) |
| Irritant reaction | 0 (0.0) | 0 (0.0) | 15 (14.3) | 0 (0.0) | 0 (0.0) |
| Weakly positive result | 1 (1.0) | 0 (0.0) | 3 (2.9) | 0 (0.0) | 0 (0.0) |
| Evidently positive result | 1 (1.0) | 0 (0.0) | 8 (7.6) | 0 (0.0) | 0 (0.0) |
| Intensely positive result | 0 (0.0) | 0 (0.0) | 2 (1.9) | 0 (0.0) | 0 (0.0) |
| Diagnosis of allergic contact dermatitis, number of patients, % | |||||
| Presence | 2 (1.9) | 0 (0.0) | 13 (12.4) | 0 (0.0) | 0 (0.0) |
| Absence | 103 (98.1) | 105 (100.0) | 92 (87.6) | 105 (100.0) | 105 (100.0) |
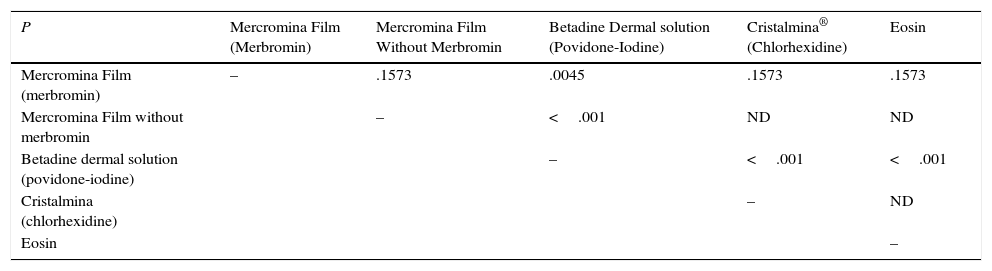
In comparison with the presence of allergic contact dermatitis according to a antiseptic pairs, the differences found between the percentage of patients who presented allergic contact dermatitis to Betadine dermal solution with respect to other antiseptics (Table 3) was statistically significant (McNemar test, P<.05). The other comparisons were not statistically significant (McNemar; P>.05). No patient presented allergic contact dermatitis to several antiseptics at the same time.
Comparison of Presence of Allergic Contact Dermatitis According to Antiseptic Pairing (McNemar Test).
| P | Mercromina Film (Merbromin) | Mercromina Film Without Merbromin | Betadine Dermal solution (Povidone-Iodine) | Cristalmina® (Chlorhexidine) | Eosin |
|---|---|---|---|---|---|
| Mercromina Film (merbromin) | – | .1573 | .0045 | .1573 | .1573 |
| Mercromina Film without merbromin | – | <.001 | ND | ND | |
| Betadine dermal solution (povidone-iodine) | – | <.001 | <.001 | ||
| Cristalmina (chlorhexidine) | – | ND | |||
| Eosin | – |
ND, no discrepancies between the two antiseptics in patch test.
Recent reviews of the use of topical antiseptics do not recommend the use of mercury derivatives, alluding to their allergenic capacity, and recommend replacing them with other safer antiseptics.4 However, in a study from 1991 by Romaguera et al.,3 merbromin induced an allergenic response in only 1%. Similarly, in our study, merbromin was shown to have a very low allergenic capacity (1.9% of patients). The differences between merbromin and other antiseptics were not statistically significant (except Betadine).
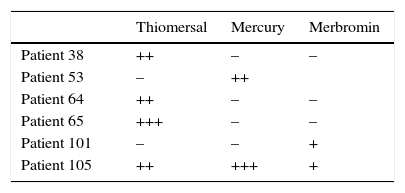
Of the 105 patients in the study, 6 had allergic contact dermatitis to mercury derivatives (5.7%) (Table 4). Of the 2 patients who obtained a positive reaction to merbromin, only 1 had concomitant allergy to thiomersal and mercury metal. Both these compounds are included in the standard GEIDAC battery.
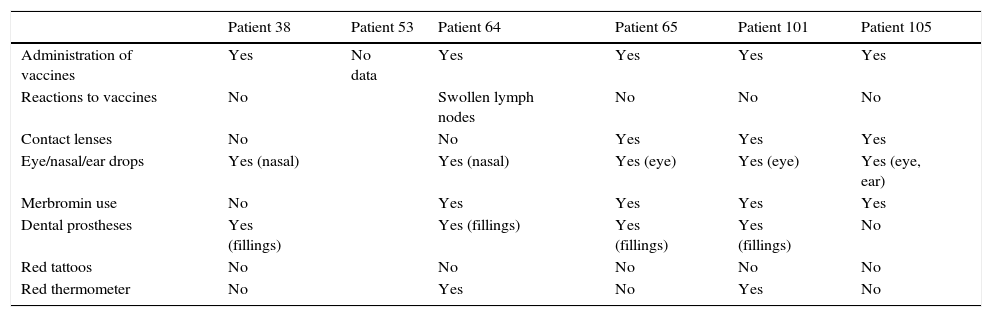
Most sensitizations to mercury are caused by exposure to sanitary products (mainly disinfectants or antiseptics) or cosmetics that have mercury compounds.5,6 For the 6 cases in our study with a positive reaction to 1 or several mercury derivatives, the potential sources of sensitization analyzed are reflected in Table 5.
Sensitization to Mercury Derivatives.
| Patient 38 | Patient 53 | Patient 64 | Patient 65 | Patient 101 | Patient 105 | |
|---|---|---|---|---|---|---|
| Administration of vaccines | Yes | No data | Yes | Yes | Yes | Yes |
| Reactions to vaccines | No | Swollen lymph nodes | No | No | No | |
| Contact lenses | No | No | Yes | Yes | Yes | |
| Eye/nasal/ear drops | Yes (nasal) | Yes (nasal) | Yes (eye) | Yes (eye) | Yes (eye, ear) | |
| Merbromin use | No | Yes | Yes | Yes | Yes | |
| Dental prostheses | Yes (fillings) | Yes (fillings) | Yes (fillings) | Yes (fillings) | No | |
| Red tattoos | No | No | No | No | No | |
| Red thermometer | No | Yes | No | Yes | No |
With regards povidone-iodine, in our study, we found 12.4% of patients with contact allergy to Betadine. These differences are statistically significant compared with other antiseptics. This percentage was much higher than expected according to the literature and might be explained by the fact that the patch tests were conducted with occlusion, as povidone-iodine has a high irritant capacity (probable false positives). Performing a repeat open application test would make it possible to differentiate between cases of irritant dermatitis and those that are truly allergic.
ConclusionIn conclusion, the prevalence of allergic contact dermatitis to merbromin in our study is very low—only 1.9%. The differences between merbromin and other antiseptics were not significant (except Betadine). This prevalence is lower than that described for other mercury derivatives (3.7%-5%) and similar to those reported for other antiseptics such as chlorohexidine (2%).
As is the case for all observational studies, the results of this study should be interpreted with caution. Although more studies are required with more patients to confirm the results, according to the findings of the present study, use of merbromin as an antiseptic is as safe as using other commonly used antiseptics.
FundingThe financial costs of the study were met by Lainco SA. These costs included those derived from the study conduct, and was well as fees paid to La Fundació Clínic per a la Recerca Biomèdica and Dr. Susana Baltá for enrolment and follow-up of patients who participated in this study.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that the procedures followed are in line with the corresponding ethics committee and the Helsinki Declaration of the World Medical Association.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors obtained the informed consent of patients and/or subjects mentioned in this article. The informed consent form is located in the archives of the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of financial or personal interest that might have an inappropriate impact on the course of this study.
Please cite this article as: Baltà Cruz S, Moreno Ribera N, Estrach Panella MT. Estudio observacional de seguridad, prospectivo y unicéntrico para determinar la capacidad alergogénica de Mercromina Film® y otros antisépticos de uso común en pacientes con dermatitis de contacto. Actas Dermosifiliogr. 2018;109:58–62.