Vitamin D plays a fundamental role in many metabolic pathways, including those involved in cell proliferation and the immune response. Serum levels of this vitamin have been linked to melanoma risk and prognosis. This study aimed to assess the prognostic value of vitamin D serum level in melanoma.
Material and methodsRetrospective, observational, longitudinal, and analytical study of 286 patients with a histologic diagnosis of melanoma in whom serum levels of vitamin D were measured at the time of diagnosis. We analyzed associations between serum level and epidemiologic and clinical variables and pathology findings; we also analyzed the influence of vitamin D on overall survival. An iterative loop was used to identify a vitamin D serum level to test for its an association with survival.
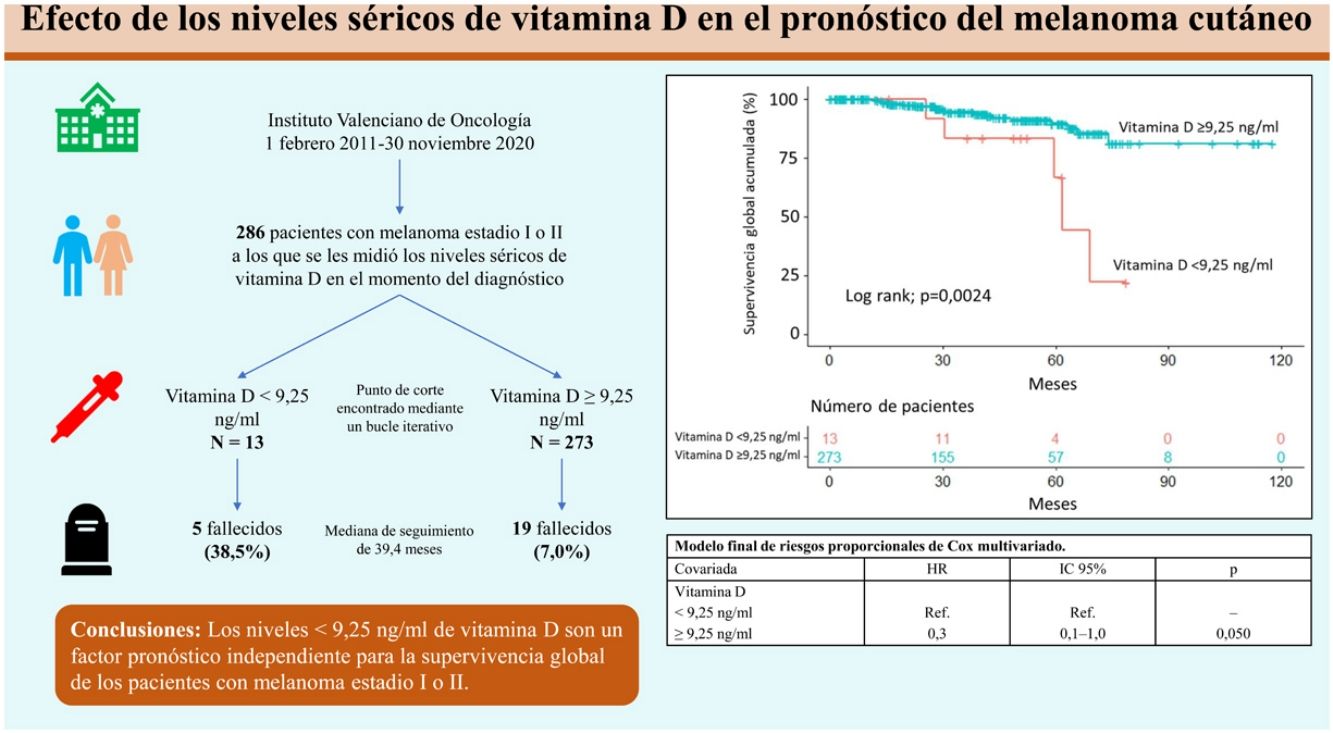
ResultsA vitamin D level less than 9.25ng/mL was associated with a histologic finding of ulceration. After a median follow-up period of 39.4 months, 24 patients (8.4%) had died. The cutoff of 9.25ng/mL was associated with lower overall survival according to both the Kaplan–Meier curves and multivariate Cox regression analysis.
ConclusionVitamin D levels less than 9.25ng/mL are associated with ulceration in melanoma and serve as an independent prognostic factor for overall survival in this disease.
La vitamina D tiene un rol fundamental en múltiples vías metabólicas, incluidas vías implicadas en la proliferación celular y la respuesta inmune. Sus niveles han mostrado una asociación con el riesgo de desarrollar el melanoma cutáneo y su pronóstico. El objetivo de este estudio fue evaluar si los niveles séricos de vitamina D influyen en el pronóstico del melanoma.
Materiales y métodosEstudio de cohorte retrospectivo, observacional, longitudinal y analítico en 286 pacientes con diagnóstico histológico de melanoma, en los que se midieron los niveles séricos de vitamina D en el momento del diagnóstico. Se analizó la relación entre los niveles de vitamina D y las características epidemiológicas, clínicas y patológicas de los pacientes, y el efecto de la vitamina D en la supervivencia global de los pacientes. Mediante un bucle iterativo se encontró el punto de corte de los niveles séricos de vitamina D de 9,25ng/mL para su relación con la supervivencia.
ResultadosUn nivel bajo de vitamina D (<9,25ng/mL) se relacionó con la ulceración en el análisis histológico. Tras una mediana de seguimiento de 39,4 meses, 24 pacientes (8,4%) fallecieron. Unos niveles de vitamina D<9,25ng/mL se asociaron con una menor supervivencia global, tanto en el análisis a través de curvas de Kaplan-Meier, como tras la regresión de Cox multivariada.
ConclusiónLos niveles<9,25ng/mL de vitamina D se asocian a la presencia de ulceración histológica en el melanoma y son un factor pronóstico independiente para la supervivencia global en estos pacientes.
Cutaneous melanoma is a clinically heterogeneous disease, with both mild and life-threatening cases. A range of clinical, histologic, and molecular factors that help predict survival have been identified.1 Many patients, however, have tumors of uncertain behavior, hence the importance of investigating other factors that could help determine prognosis.2
The 2 main forms of vitamin D that are important to humans are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). While they can both be obtained from diet, 90% of vitamin D3 is obtained via skin exposure to UV radiation from the sun.3 Excessive exposure, however, contributes to skin aging and favors the development of skin cancer. It is not surprising thus that patients with melanoma are often found to have low vitamin D levels during follow-up, as they tend to take extreme measures to protect themselves from the sun.4 Vitamin D may be involved in the natural history of cancer because it supports the proper function of the immune system and also has a role in cell division, where it induces differentiation and apoptosis and inhibits cell proliferation and angiogenesis in both normal and cancer cells.5
Vitamin D levels and germline vitamin D receptor polymorphisms could have a pathogenic role in cutaneous melanoma.5–9 Previous studies have suggested that vitamin D might be of prognostic value in melanoma, with low levels indicating potentially shorter survival, independently of well-known poor prognosis factors.10–16 The studies to date, however, have used highly heterogeneous methods, especially in terms of how vitamin D serum levels are measured.
The aim of this study was to evaluate the prognostic value of vitamin D serum levels in a retrospective cohort of patients with cutaneous melanoma, with adjustment for key prognostic markers and use of a specific statistical methodology to select an optimal vitamin D serum cutoff associated with survival.
Materials and methodsStudy designWe conducted a retrospective, observational, longitudinal, analytical cohort study of patients with a histologic diagnosis of localized (stage I and II) melanoma and data on vitamin D serum level at the time of diagnosis. The patients were seen at Instituto Valenciano de Oncología in Valencia, Spain, between February 1, 2011 and November 30, 2020. All the patients provided informed consent for inclusion in this study.
In order to evaluate the prognostic value of vitamin D serum level, the patients were divided into 2 groups: those with a level <9.25ng/mL and those with a higher level. This cutoff of 9.25ng/mL was identified using an iterative loop, described in the statistical analysis section.
Independent variables were sex, age (<65 years vs. ≥65 years), tumor location (head/neck vs. upper extremity vs. trunk vs. lower extremity vs. acral site), histologic subtype (lentigo maligna melanoma [LMM] vs. superficial spreading melanoma [SSM] vs. nodular melanoma [NM] vs. acral lentiginous melanoma [ALM] vs. other/unspecified), Breslow thickness (≤2mm vs. >2mm), mitotic rate (≤5mitoses/mm2 vs. >5mitoses/mm2), and a histologic finding of ulceration.
The cutoff for age was identified in the final multivariate Cox regression model; the cutoffs used for Breslow thickness and mitotic rate were pre-established by the researchers.
Overall survival, defined as time from primary tumor excision to death, was used as the dependent variable to assess the prognostic value of vitamin D serum level. Patient status was updated at the end of the study (April 30, 2021) by consulting the national death registry.
Statistical analysisContinuous variables are expressed as mean (SD) for normally distributed variables and median (interquartile range [IQR]) otherwise. Normality of distribution was checked using the Kolmogorov–Smirnov test Qualitative variables are expressed as absolute and relative frequencies.
Differences in the distributions of each variable according to vitamin D level (<9.25ng/mL vs. ≥9.25ng/mL) were evaluated using contingency tables. The groups were compared using the Pearson χ2 test or, when the expected count was less than 5 in at least 1 cell in the 2×2 tables, the Fisher exact test.
Potential vitamin D cutoff points (each quarter of a unit for measured levels at time of diagnosis) were tested using an iterative loop in which patients were split into 2 groups to test for significant differences in survival. These differences were tested using the log-rank test. The cutoff showing the most significant difference between groups (9.25ng/mL) was chosen and used to build the corresponding Kaplan–Meier survival curves. Univariate and multivariate Cox proportional hazards models were built using stepwise backward selection to evaluate the association between vitamin D serum level and overall survival. Finally, Schoenfeld residuals were used to check the proportional hazards assumption. The results determined that age, which was considered a continuous variable in the initial model, should be entered as a categorical variable (<65 years vs. ≥65 years) in the final version. Statistical analyses were conducted in IBM SPSS version 20.0 and R Studio version 4.1.0. All P values were 2-tailed and values less than .05 were considered statistically significant.
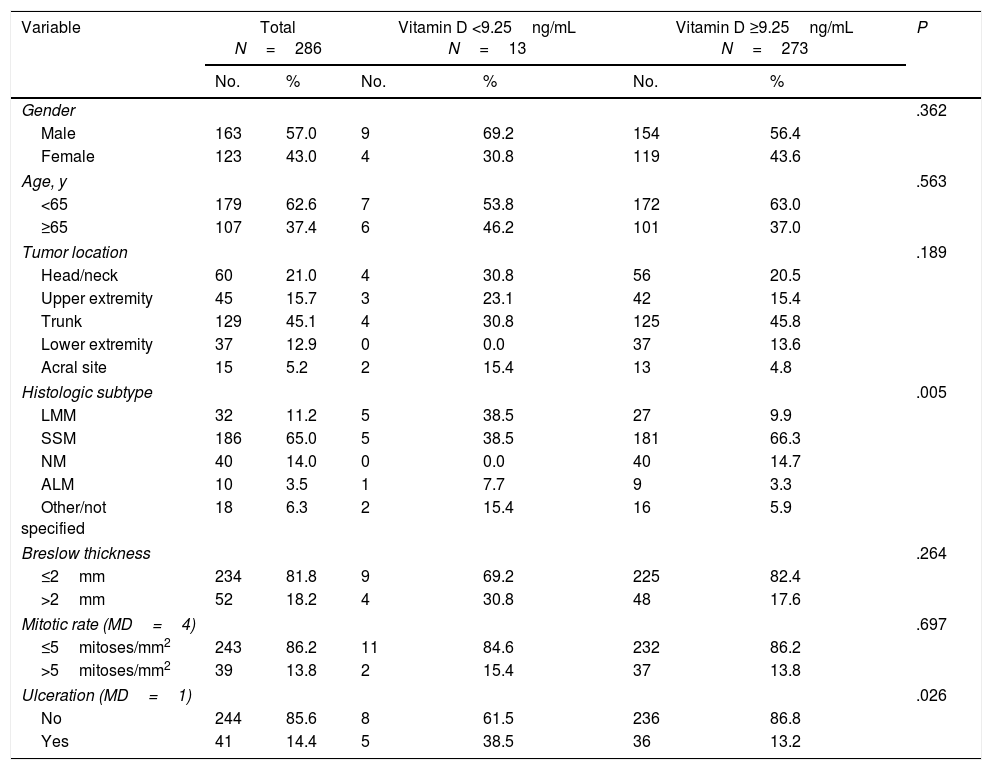
ResultsThe patients’ characteristics according to vitamin D level are shown in Table 1. We studied 286 patients, mostly men (57%), with a median age of 57.5 years (IQR, 45.75–70 years). The most common tumor was an SMM (186, 65%) located on the trunk (129, 45.1%) with a Breslow thickness ≤2mm (234, 81.8%), a mitotic rate ≤5/mm2 (243, 86.2%), and no ulceration (85.6%)
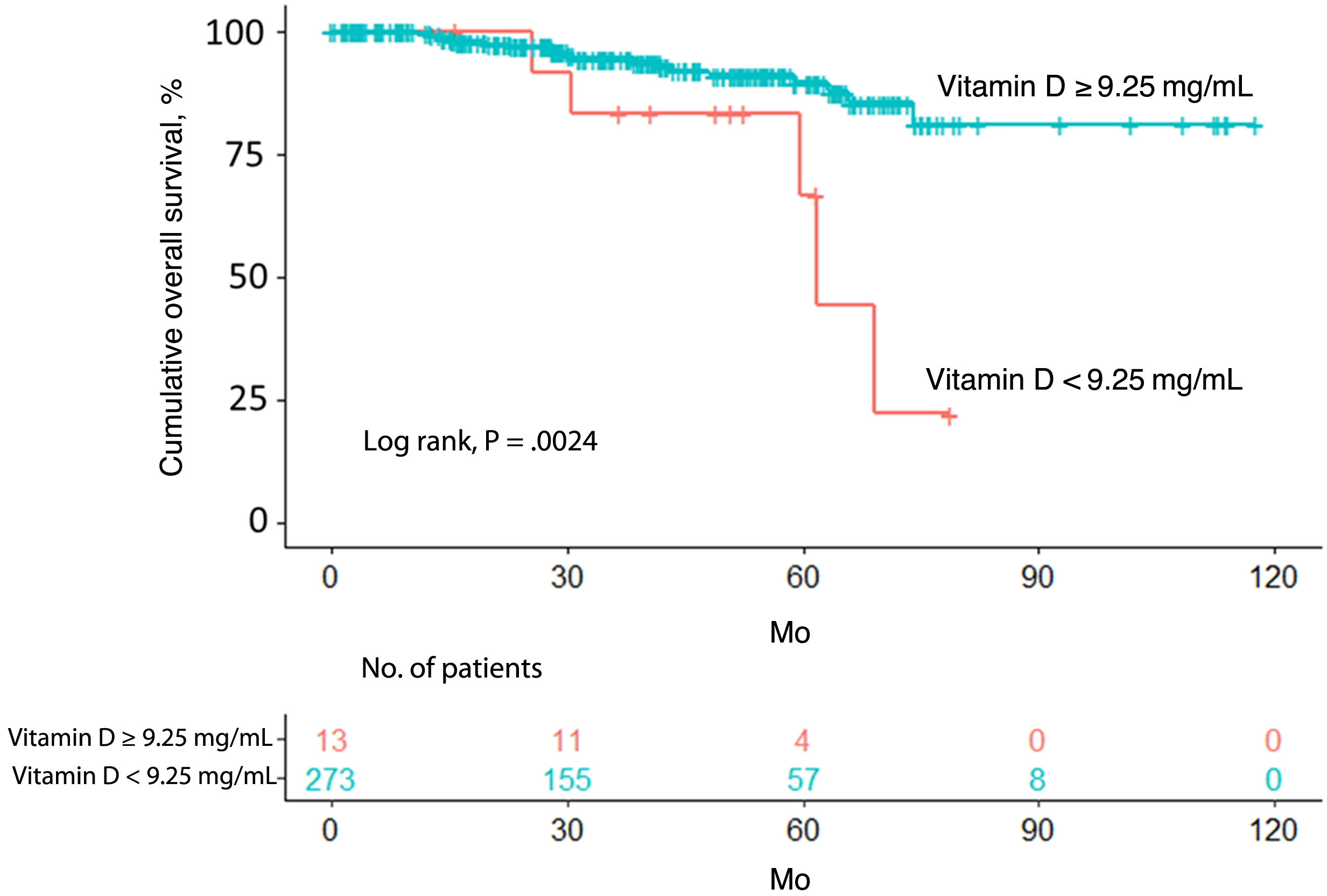
Study population and contingency tables showing distribution of variables according to vitamin D level.
| Variable | Total N=286 | Vitamin D <9.25ng/mL N=13 | Vitamin D ≥9.25ng/mL N=273 | P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Gender | .362 | ||||||
| Male | 163 | 57.0 | 9 | 69.2 | 154 | 56.4 | |
| Female | 123 | 43.0 | 4 | 30.8 | 119 | 43.6 | |
| Age, y | .563 | ||||||
| <65 | 179 | 62.6 | 7 | 53.8 | 172 | 63.0 | |
| ≥65 | 107 | 37.4 | 6 | 46.2 | 101 | 37.0 | |
| Tumor location | .189 | ||||||
| Head/neck | 60 | 21.0 | 4 | 30.8 | 56 | 20.5 | |
| Upper extremity | 45 | 15.7 | 3 | 23.1 | 42 | 15.4 | |
| Trunk | 129 | 45.1 | 4 | 30.8 | 125 | 45.8 | |
| Lower extremity | 37 | 12.9 | 0 | 0.0 | 37 | 13.6 | |
| Acral site | 15 | 5.2 | 2 | 15.4 | 13 | 4.8 | |
| Histologic subtype | .005 | ||||||
| LMM | 32 | 11.2 | 5 | 38.5 | 27 | 9.9 | |
| SSM | 186 | 65.0 | 5 | 38.5 | 181 | 66.3 | |
| NM | 40 | 14.0 | 0 | 0.0 | 40 | 14.7 | |
| ALM | 10 | 3.5 | 1 | 7.7 | 9 | 3.3 | |
| Other/not specified | 18 | 6.3 | 2 | 15.4 | 16 | 5.9 | |
| Breslow thickness | .264 | ||||||
| ≤2mm | 234 | 81.8 | 9 | 69.2 | 225 | 82.4 | |
| >2mm | 52 | 18.2 | 4 | 30.8 | 48 | 17.6 | |
| Mitotic rate (MD=4) | .697 | ||||||
| ≤5mitoses/mm2 | 243 | 86.2 | 11 | 84.6 | 232 | 86.2 | |
| >5mitoses/mm2 | 39 | 13.8 | 2 | 15.4 | 37 | 13.8 | |
| Ulceration (MD=1) | .026 | ||||||
| No | 244 | 85.6 | 8 | 61.5 | 236 | 86.8 | |
| Yes | 41 | 14.4 | 5 | 38.5 | 36 | 13.2 | |
Abbreviation: MD, missing data.
Thirteen patients (4.5%) had a vitamin D serum level <9.25ng/mL while 273 (95.5%) had a level above this cutoff.
Tumor location varied significantly according to vitamin D level. Patients in the lower cutoff group were significantly more likely than those with a level ≥9.25ng/mL to have LMM or ALM and significantly less likely to have SSM or NM (P=.005) (Table 1). Ulceration was also more common in these patients (38.5% vs. 13.2%, P=.026) (Table 1).
No significant between-group differences were found for sex, age, location, Breslow thickness, or mitotic rate.
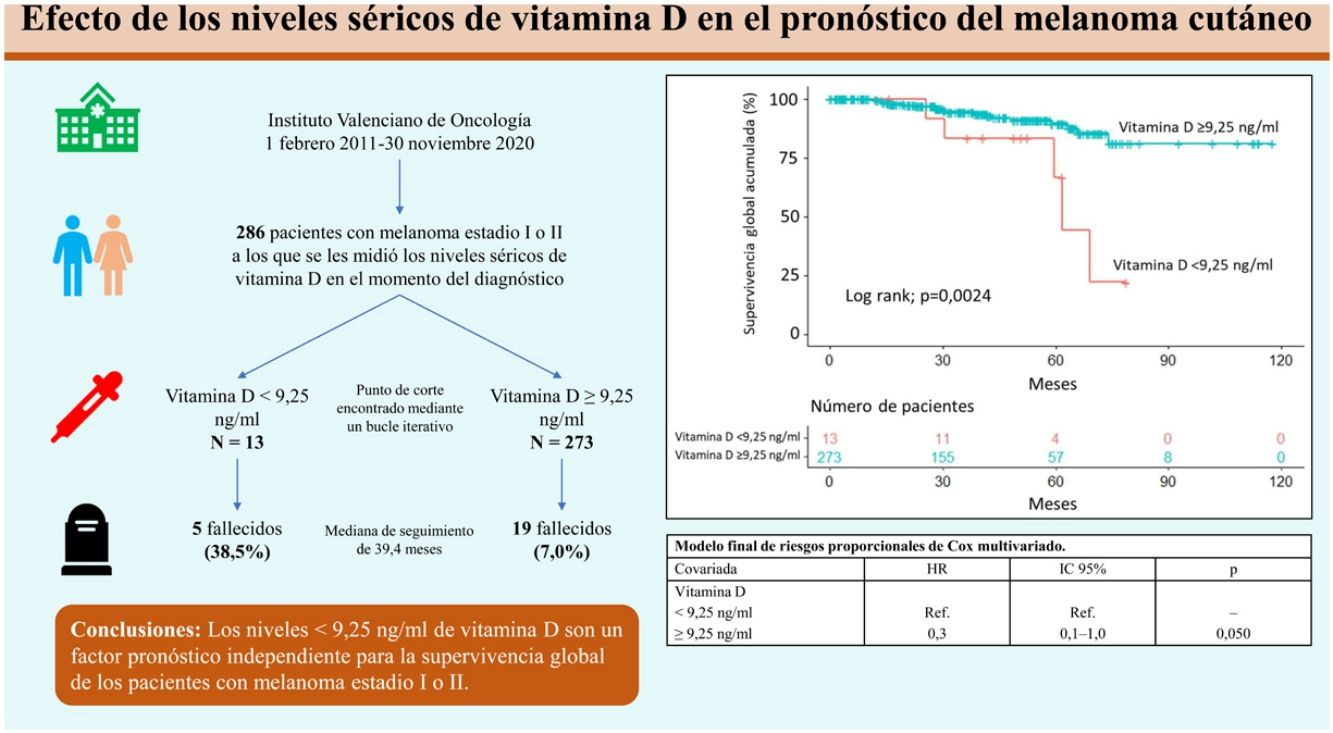
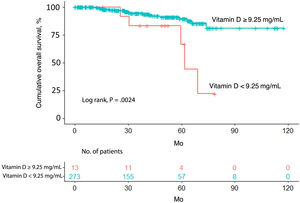
Overall survivalTwenty-four patients (8.4%) died over a median follow-up of 39.4 months. Of these, 5 had a vitamin D level <9.25ng/mL (5/13, 38.5%) and 19 had a level ≥9.25ng/mL (19/273, 7.0%). Overall survival was significantly different between the 2 groups (P=.0024) (Fig. 1).
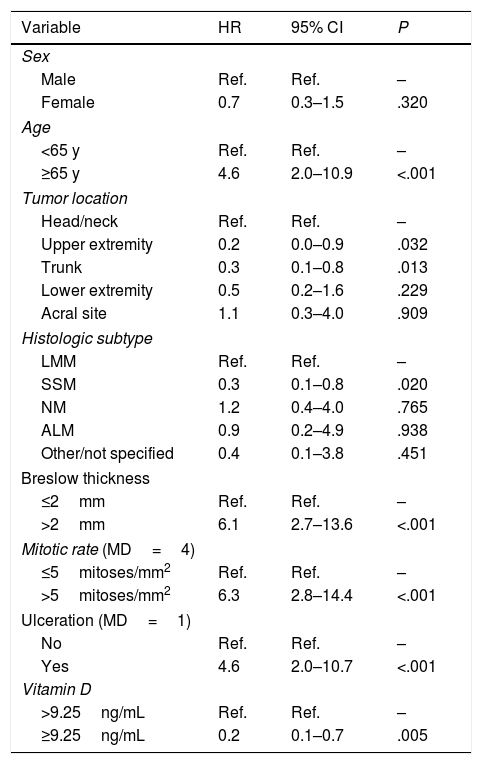
The univariate Cox regression model confirmed that patients with a vitamin D level <9.25ng/mL were more likely to have worse overall survival than those with a level above the cutoff (hazard ratio [HR]=0.2; 95% CI, 0.1–0.7; P=.005). Worse survival was also observed in patients aged 65 years or older (HR=4.6; 95% CI, 2.0–10.9; P<.001) and patients with a Breslow thickness >2mm (HR=6.1; 95% CI, 2.7–13.6; P<.001), a mitotic rate >5mitoses/mm2 (HR=6.3; 95% CI, 2.8–4.4; P<.001), and ulceration (HR=4.6; 95% CI, 2.0–10.7; P<.001). Location on the upper extremities or trunk was associated with better overall survival ([upper extremity vs. head/neck: HR=0.2; 95% CI, 0.0–0.9; P=.032]; [trunk vs. head/neck: HR=0.3; 95% CI, 0.1–0.8; P=.013]). Better survival was also observed in patients with SSM versus LMM (HR=0.3; 95% CI, 0.1–0.8; P=.020) (Table 2).
Univariate Cox proportional hazards model.
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Sex | |||
| Male | Ref. | Ref. | – |
| Female | 0.7 | 0.3–1.5 | .320 |
| Age | |||
| <65 y | Ref. | Ref. | – |
| ≥65 y | 4.6 | 2.0–10.9 | <.001 |
| Tumor location | |||
| Head/neck | Ref. | Ref. | – |
| Upper extremity | 0.2 | 0.0–0.9 | .032 |
| Trunk | 0.3 | 0.1–0.8 | .013 |
| Lower extremity | 0.5 | 0.2–1.6 | .229 |
| Acral site | 1.1 | 0.3–4.0 | .909 |
| Histologic subtype | |||
| LMM | Ref. | Ref. | – |
| SSM | 0.3 | 0.1–0.8 | .020 |
| NM | 1.2 | 0.4–4.0 | .765 |
| ALM | 0.9 | 0.2–4.9 | .938 |
| Other/not specified | 0.4 | 0.1–3.8 | .451 |
| Breslow thickness | |||
| ≤2mm | Ref. | Ref. | – |
| >2mm | 6.1 | 2.7–13.6 | <.001 |
| Mitotic rate (MD=4) | |||
| ≤5mitoses/mm2 | Ref. | Ref. | – |
| >5mitoses/mm2 | 6.3 | 2.8–14.4 | <.001 |
| Ulceration (MD=1) | |||
| No | Ref. | Ref. | – |
| Yes | 4.6 | 2.0–10.7 | <.001 |
| Vitamin D | |||
| >9.25ng/mL | Ref. | Ref. | – |
| ≥9.25ng/mL | 0.2 | 0.1–0.7 | .005 |
Abbreviations: HR, hazard ratio; MD, missing data.
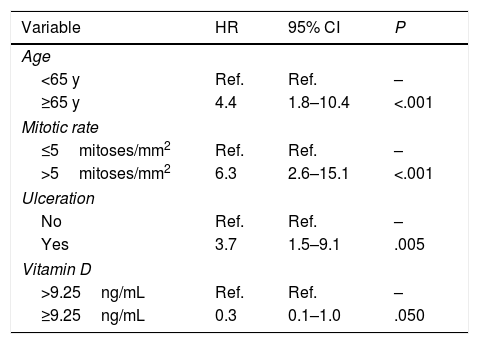
The variables included in the final multivariate model were age (≥65 years vs. <65 years; HR=4.4; 95% CI, 1.8–10.4; P<.001), mitotic rate (>5mitoses/mm2 vs. ≤5mitoses/mm2; HR=6.3 95% CI, 2.6–15.1; P<.001), ulceration (yes vs. no; HR=3.7; 95% CI, 1.5–9.1; P=.005), and vitamin D level (≥9.25ng/mL vs. <9.25ng/mL; HR=0.3; 95% CI, 0.1–1.0; P=.050). Age, mitotic rate, ulceration, and vitamin D level were independent predictors of overall melanoma survival in the multivariate analysis (Table 3).
Final multivariate Cox proportional hazards model.a
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age | |||
| <65 y | Ref. | Ref. | – |
| ≥65 y | 4.4 | 1.8–10.4 | <.001 |
| Mitotic rate | |||
| ≤5mitoses/mm2 | Ref. | Ref. | – |
| >5mitoses/mm2 | 6.3 | 2.6–15.1 | <.001 |
| Ulceration | |||
| No | Ref. | Ref. | – |
| Yes | 3.7 | 1.5–9.1 | .005 |
| Vitamin D | |||
| >9.25ng/mL | Ref. | Ref. | – |
| ≥9.25ng/mL | 0.3 | 0.1–1.0 | .050 |
Abbreviation: HR, hazard ratio.
In this study of 286 patients with melanoma, a vitamin D serum level <9.25ng/mL was associated with worse overall survival, independently of Breslow thickness and ulceration(2 key prognostic factors) and other known factors such as mitotic rate and age.1
Our findings are consistent with previous reports, summarized in a recent meta-analysis.13 Studies to date, however, have used highly heterogeneous methods, particularly to measure serum vitamin D levels.
In their first study of the association between vitamin D and melanoma, Newton-Bishop et al.10 found an association between low levels and worse recurrence-free and overall survival, even after adjustment for Breslow thickness. They analyzed vitamin D levels as a continuous variable and a categorical variable based on tertiles calculated using values reported for the study population. In addition, blood sampling was performed 3–6 months after diagnosis.10
In a second study, the same group observed worse melanoma-specific survival in patients with a low vitamin D level at diagnosis (<20nmol/L, which is equivalent to <8.01ng/mL [conversion factor, 2.496]).15 They also observed an improvement in survival for each 10-nmol/L increase, as apart from considering vitamin D as a continuous variable, they also analyzed it by categories (<20, 20–60, 60–85, 85–100, and 100+ nmol/L). Similarly to in the first study, they adjusted for Breslow thickness but not ulceration.
Timerman et al.12 reported worse melanoma-specific mortality in patients with a low vitamin D serum level, particularly in those with metastatic melanoma. They used the standard cutoff of 20ng/mL, which is the established level for adequate bone metabolism. Blood sampling was performed at different times during follow-up. No adjustments were made for Breslow thickness or tumor ulceration.12
In the study by Weinsten et al.,16 season-specific vitamin D levels categorized by quantiles were associated with worse prognosis in melanoma following adjustment for age.
Bade et al.14 observed shorter overall survival in patients with vitamin D serum levels in the lowest quartile compared with those in the top quartile. Only univariate Kaplan–Meier curves, however, were used.
Unlike the above authors, Saiag et al.17 found no differences in overall survival between patients with a low vitamin D level at diagnosis, although they did find that variations over time (both increases and decreases from baseline) had prognostic value. Similarly to the other authors, they used quartile values as cutoff points. They adjusted for both Breslow thickness and ulceration.17
Fang et al.11 used the most similar methodology to ours. They used recursive partitioning to identify the split associated with the lowest P value in the log-rank test. On applying the cutoff identified—16ng/mL—they found that patients with lower levels had significantly worse melanoma-specific, disease-free, and overall survival after adjustment for logarithmic C-reactive protein, age, sex, clinical stage, and blood draw season in the multivariate model.
We identified a cutoff of 9.25ng/mL in our study using an iterative loop and log-rank testing. This methodology, while different to that of Fang et al.,11 enabled us to identify the cutoff showing the most significant survival difference between groups. Another methodological difference was that we adjusted for Breslow thickness and tumor ulceration rather than clinical stage (III/IV vs. I/II) in the multivariate model. We also adjusted for mitotic rate. While this factor is no longer used to determine clinical stage in the latest melanoma staging system of the American Joint Committee on Cancer,’ it is still an important determinant of prognosis.1
Methodologies such as that used in the present study are interesting because most studies to date have used reference values related to bone metabolism to analyze vitamin D levels. These values, however, may not necessarily correlate with prognosis in cutaneous melanoma. The prognostic value of vitamin D in melanoma needs to be studied by analyzing levels as a continuous variable across a wide range of measurements and in larger studies.
While our findings show a direct association between vitamin D serum levels and prognosis in melanoma, they also point to an indirect association. They showed, for example, a link between low vitamin D levels (<9.25ng/mL) and ulceration. Previous studies have reported associations between low levels and greater Breslow thickness,10,11,14,17–19 a higher mitotic rate,18,20 a higher clinical stage,11,17 and ulceration.11,15,17,18,20 Bade et al.14 found that older melanoma patients had lower vitamin D levels, although other studies have reported an inverse relationship.11,12
Neither our findings nor those of previous studies indicate a cause-effect relationship between vitamin D serum level and survival in cutaneous melanoma. Rather, they show a direct and indirect correlation between low levels and prognosis. More prospective studies and clinical trials are needed to investigate the impact of vitamin D supplementation in this setting.
The strengths of this study include the prospective recording of data, adjustment for key prognostic factors in melanoma, and the use of iterative looping to determine the vitamin D cutoff level most closely associated with survival.
Limitations include the small number of patients in certain categories and the considerable difference in the size of the 2 groups (95.5% of patients had a vitamin D level≥9.25ng/mL).
In conclusion, vitamin D serum levels <9.25ng/mL are associated with worse prognosis in cutaneous melanoma. More studies analyzing larger samples and vitamin D levels as a continuous variable are needed to identify a more accurate cutoff associated with survival in this setting and to examine the usefulness of vitamin D measurements in routine clinical practice.
Conflicts of interestThe authors declare that they have no conflicts of interest.