This is the case of a 92-year-old woman who presented to our center with a 2-year history lesion on the second finger of her right hand.
On physical examination, a 3cm exophytic bleeding tumor was identified on the dorsum of the second finger of the hand, which did not seem to infiltrate deep planes and uniformly widened the finger. The lesion was resected, and the specimen was sent to Pathology Unit.
The macroscopic study revealed a 3cm × 1.5cm × 1cm lesion—light brown in color and of a multinodular morphology—with two 0.7cm and 0.5cm whitish regions of stony consistency.
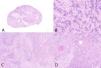
Histologically, an epithelial proliferation corresponding to a moderately differentiated squamous cell carcinoma with presence of keratin whorls and keratin pearls was identified, without regions of normal epidermis (fig. 1). This region showed a squamous immunophenotype with positivity for p40, p63, CK AE1/AE3, and CK 5/6, while SATB2 (fig. 2), actin, and desmin tested negative; vimentin tested positive in the osteoblastic component and negative in the squamous one.
In the transition to the described area, a well-demarcated region of a different morphology was observed, revealing areas of eosinophilic matrix and basophilic regions with calcifications resembling osteoid deposition. In this regions, large cells occupying the spaces between trabeculae and osteoid deposits were observed. Cytologically, they showed vesicular nuclei with a prominent nucleolus and numerous–some even atypical–mitoses (fig. 1).
These neoplastic cells were intensely positive for nuclear SATB2 and negative for CKAE1/AE3 (fig. 2), CK5/6, p40, p63, actin, and desmin. A diagnosis of moderately differentiated squamous cell carcinoma with osteoblastic osteosarcoma differentiation was established. No lymphovascular or perineural invasion was reported.
One year later, the patient is still being monitored by the Dermatology Unit without any local or distant relapses.
Cutaneous squamous cell carcinoma with sarcomatoid differentiation is a very rare malignant tumor, which is more common in organs such as the uterus, breast, bladder, liver, and lungs, and is associated with a very aggressive clinical behavior. This skin neoplasm usually occurs in elderly patients, with similar frequency in men and women, in the head and neck region.1–4
Histologically, it is characterized by a clearly biphasic pattern, with presence of an epithelial component and a mesenchymal component. Regarding the former, the literature refers to its association predominantly with basal cell carcinoma, with presence of squamous cell carcinoma or cutaneous adnexal neoplasms such as porocarcinoma, proliferating cystic trichilemmal tumor, or spiradenocarcinoma being less frequent.5
The most common mesenchymal component is osteosarcoma6; however, cases with presence of chondrosarcoma, rhabdomyosarcoma, angiosarcoma,7 fibrosarcoma, malignant fibrous histiocytoma, and, much less commonly, liposarcoma or neurofibrosarcoma have also been reported.1–4
Although immunohistochemically, sarcomatoid squamous cell carcinoma can show positivity for SATB2, this does not mean that it is an osteosarcoma.8
The histogenesis of carcinosarcomas is still unknown, and currently, there are 2 theories that try to explain it.2–4,9,10 First, the multiclonal theory suggests that carcinosarcomas are collision tumors, defending the existence of 2 components that arise independently from 2 or more different totipotent stem cells. This theory is supported by the fact that the morphological and histochemical findings of both tumor components do not show any similarities. The other theory states that the origin of these tumors is monoclonal and stands on a genetic basis, as studies have confirmed the presence of a chromosomal changes in the 2 components.
Unlike visceral carcinosarcomas, cutaneous forms are not as well characterized due to their low incidence rate. Therefore, their differential diagnosis can become complicated and includes entities such as squamous cell melanoma, dermatofibrosarcoma protuberans, and sarcoma metastases from a different location.
It is known that their prognosis will depend, in part, on the nature of the epithelial component, usually being more favorable in cases with presence of basal cell carcinoma, while the epidermal component of epidermoid or adnexal carcinoma has been associated with a grim prognosis and a higher recurrence rate4. In carcinosarcomas with an adnexal component, the incidence rate of local metastasis (19%), affected lymph nodes (19%), and visceral metastasis (26%) is higher, and they are associated with a 50% disease-free survival at 5 years vs 70% for those with a basal cell component. The survival rate reported of cases with a squamous cell carcinoma component is similar to the survival rate of those with an adnexal component. Since the median follow-up time reported is short (12 months), there may be a reporting bias, meaning that the true long-term prognosis of cutaneous carcinosarcoma is not yet fully defined.4
Other factors that have been associated with a worse prognosis are the lesion being of rapid and recent growth, the presence of nodal metastasis, a long history of skin cancer, and predominance of young patients.1,4,9 On the other hand, no prognostic differences were found based on the location of the tumor or the mesenchymal component.4
Regarding treatment, it has been reported that in cases in which the lesion is limited to the skin, surgical excision is the treatment of choice. However, given the aggressiveness and likelihood of recurrence and metastasis, close patient follow-up is advised.1–3
In conclusion, primary cutaneous carcinosarcoma is a rare tumor whose diagnosis has clinical, therapeutic, and prognostic implications because it is a malignant neoplasm. Although we still don’t know its natural history, its behavior is aggressive, and excision of the lesion and close follow-up of these patients are advised for the early detection of metastasis and/or recurrences.
Conflicts of interestNone declared.