Immunosuppressants and immunomodulators are widely used in dermatology. Some of these drugs, however, can increase the risk of severe COVID-19. New antivirals against SARS-CoV-2 have been shown to reduce progression to COVID-19 pneumonia in susceptible patients, but their availability is limited. On May 23, 2022, the Spanish Agency for Medicines and Medical Devices (AEMPS) updated its priority eligibility criteria for SARS-CoV-2 antiviral therapy. In this practical guide, we review the indications for these new drugs and provide guidance on which patients with mild to moderate COVID might benefit from their use in dermatology.
En Dermatología es frecuente el uso de inmunosupresores e inmunomoduladores, algunos de los cuales pueden predisponer al desarrollo de enfermedad grave por SARS-CoV-2. Las nuevas terapias antivirales frente al SARS-CoV-2 han demostrado reducir la progresión a neumonía por COVID-19 grave en pacientes susceptibles. El pasado 23 de mayo, la Agencia Española de Medicamentos y Productos Sanitarios publicó la última actualización sobre los criterios para la priorización en el acceso precoz a estos fármacos debido a su limitada disponibilidad. En esta guía práctica revisamos los pacientes dermatológicos que en caso de contraer COVID-19 leve-moderada pueden beneficiarse de los nuevos antivirales, así como su indicación.
Several antivirals are available for the treatment of SARS-CoV-2 infection, but their availability is limited. On May 23, 2022, the Spanish Agency for Medicines and Medical Devices (AEMPS) updated its priority eligibility criteria for SARS-CoV-2 antiviral therapy.1 In this practical guide, we review the indications for these drugs and provide guidance on which dermatology patients with mild to moderate COVID-19 could benefit from their use.
Immunosuppressants and immunomodulators are widely used in dermatology, but a number of these drugs may increase the risk of severe COVID-19.2,3 New antivirals have been shown to reduce progression to severe COVID in patients on immunomodulators classified as high risk.4 The following immunomodulators, all frequently used in dermatology, have been classified as high risk by the AEMPS (Table 1): high-dose or long-term corticosteroids; immunosuppressive drugs administered in the previous 3 months; and certain immunomodulatory biologics administered in the previous 3 months (or previous 6 months in the case of anti-CD20 therapy). Patients on immune checkpoint inhibitors and other cancer treatments that do not increase infection risk, such as targeted therapy drugs for melanoma and hedgehog pathway inhibitors, are not eligible for new antivirals according to the AEMPS priority criteria (Table 1). Finally, there are many immunomodulatory drugs commonly used in dermatology on which the AEMPS has not taken a position (Table 2). Evidence of their safety in COVID is scarce, although reports to date do not seem to indicate an increased risk of progression to severe disease. Some authors have even proposed that some of these drugs may help curb the cytokine storm induced by the virus.5,6
Immunomodulators Commonly Used in Dermatology Classified by Risk According to the Spanish Agency for Medicines and Medical Devices (AEMPS).
| High risk | Treatment with oral corticosteroids (prednisolone) in any of the following regimens in previous 30 days:• ≥10mg/d for >4 consecutive weeks• ≥10mg/d for >10 consecutive weeks• ≥10mg/d for >7 consecutive days |
| Treatment with nonbiologic immunomodulators in previous 3 months:• Oral or subcutaneous methotrexate >20mg/wk or >15mg/m2/wk• 6-Mercaptopurine>1.5mg/kg/d• Azathioprine>3mg/kg/d• Cyclosporine• Mycophenolate• Tacrolimus• Sirolimus• Everolimus | |
| Treatment with any of the following immunomodulatory biologics in previous 3 months (or 6 months in the case of anti-CD20 therapies):• Anti-CD20 monoclonal antibodies: rituximab• Anti-CCR4 monoclonal antibodies: mogamulizumab• T-cell inhibition fusion proteins: abatacept• Interleukin 1 inhibitors: anakinra, canakinumab, and rilonacept• Anti-CD52 monoclonal antibodies: alemtuzumab• Protein kinase inhibitors: imatinib• Janus kinase inhibitors: tofacitinib, baricitinib, upadacitinib | |
| Low risk | • Immune checkpoint inhibitors: pembrolizumab, nivolumab, avelumab, cemiplimab• Targeted therapy drugs: dabrafenib-trametinib, vemurafenib-cobimetinib, encorafenib-binimetinib• Hedgehog pathway inhibitors: vismodegib, sonidegib |
Immunomodulatory Drugs Used in Dermatology That Have Not Been Classified by the Spanish Agency for Medicines and Medical Devices (AEMPS).
| Immunomodulatory drugs | Target |
|---|---|
| Adalimumab, etanercept, infliximab, certolizumab | TNF-α |
| Dupilumab | Interleukin 4/13 |
| Tocilizumab | Interleukin 6 |
| Ustekinumab | Interleukin 12/23 |
| Ixekizumab, secukinumab, brodalumab | Interleukin 17 |
| Guselkumab, tildrakizumab, risankizumab | Interleukin 23 |
| Omalizumab | Immunoglobulin E |
| Apremilast | Phosphodiesterase 4 |
New treatment options for SARS-CoV-2 infection can be divided into 2 classes (Table 3): antivirals and monoclonal antibodies. Antivirals include nirmatrelvir/ritonavir (Paxlovid), remdesivir (Veklury), and molnupiravir (Lagevrio), which block enzymes that are crucial to viral replication.7–9 Monoclonal antibodies include casirivimab/imdevimab (Ronapreve) and sotrovimab (Xevudy), which bind to different epitopes on the spike protein of SARS-CoV-2, preventing the virus from entering human cells.4,10 These drugs are indicated for all patients with mild to moderate COVID-19 who do not require hospital admission and who are being treated with any of the high-risk immunomodulators mentioned, regardless of vaccination status.
New Treatment Options for SARS-CoV-2 Infection.
| Drug | Type | Time of administration | Administration route | Dosage |
|---|---|---|---|---|
| Nirmatrelvir/ritonavirPaxlovid | Antiviral | First 5 days | Oral | 300mg nirmatrelvir+100mg ritonavir every 12h, 5 d |
| RemdesivirVeklury | Antiviral | First 7 days | Intravenous | Day 1: 200mgDays 2 and 3: 100mg |
| Molnupiravir(Lagevrio) | Antiviral | First 5 days | Oral | 800mg every 12h, 5 d |
| Casirivimab/imdevimabRonapreve | Monoclonal antibodies | First 7 days | Intravenous or subcutaneous | 600mg casirivimab+600mg imdevimab, single dose |
| SotrovimabXevudy | Monoclonal antibodies | First 5 days | Intravenous | 500mg, single dose |
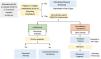
For adults (Fig. 1), the AEMPS recommends nirmatrelvir/ritonavir for 5 days as the treatment of choice because it is effective, easy to prescribe, and readily accessible. Drug-drug interactions are the main contraindication, as ritonavir is a strong inhibitor of cytochrome CYP3A.7 Choice of drug for patients in whom nirmatrelvir/ritonavir is contraindicated (Table 4) depends on immunoglobulin G titers against the SARS-CoV-2 spike protein. Monoclonal antibodies are the first-line option for patients with a titer of less than 260 binding antibody units per milliliter, while antivirals are indicated for patients with higher titers or for whom serology is not available. Choice of monoclonal antibody depends on the SARS-CoV-2 variant suspected. When omicron is suspected, the treatment of choice is sotrovimab, the only monoclonal antibody to have shown in vitro neutralizing activity against this variant.11 In other cases, casirivimab/imdevimab is used. The antiviral of choice is remdesivir. When this is not available, molnupiravir, an unauthorized drug with a use recommendation from the Committee for Medicinal Products for Human Use, can be used. Monoclonal antibodies and antivirals should both be started within 5 to 7 days of symptom onset.
Drug Interactions with Paxlovid (Based on Summary of Product Characteristics).
| Main active ingredients that are contraindicated with paxlovid | |||
| Fusidic acid | Diazepam | St. John's wort | Propoxyphene |
| Alfuzosin | Dihydroergotamine | Lomitapide | Quetiapine |
| Amiodarone | Dronedarone | Lovastatin | Quinidine |
| Astemizole | Encainide | Lurasidone | Ranolazine |
| Avanafil | Ergonovine | Methylergonovine | Rifampicin |
| Bepridil | Ergotamine | Midazolam oral | Sildenafil |
| Carbamazepine | Estazolam | Neratinib | Simvastatin |
| Cisapride | Phenytoin | Pethidine | Terfenadine |
| Clorazepate | Phenobarbital | Pimozide | Triazolam |
| Clozapine | Flecainide | Piroxicam | Vardenafil |
| Colchicine | Flurazepam | Propafenone | Venetoclax |
| Main active ingredients that require close monitoring | |||
| Abemaciclib | Delamanid | Itraconazole | Risperidone |
| Afatinib | Dexamethasone | Ketoconazole | Rivaroxaban |
| Alprazolam | Desipramine | Lamotrigine | Rosuvastatin |
| Amitriptyline | Digoxin | Levothyroxine | Salmeterol |
| Amlodipine | Diltiazem | Loratadine | Sertraline |
| Amphetamine | Divalproex | Maraviroc | Sulfamethoxazole/ |
| Apalutamide | Efavirenz | Methadone | Trimethoprim |
| Atorvastatin | Encorafenib | Parenteral midazolam | Tacrolimus |
| Atovaquone | Erythromycin | Morphine | Tadalafil |
| Bedaquiline | Ethinylestradiol | Nifedipine | Theophylline |
| Bosentan | Everolimus | Nilotinib | Thioridazine |
| Budesonide | Fentanyl | Norbuprenorphine | Triamcinolone |
| Buprenorphine | Fexofenadine | Nortriptyline | Vinblastine |
| Bupropion | Fluoxetine | Paroxetine | Vincristine |
| Buspirone | Fostamatinib | Prednisolone | Vorapaxar |
| Ceritinib | Glecaprevir/pibrentasvir | Propionate fluticasone | Voriconazole |
| Ciclosporin | Haloperidol | Raltegravir | Warfarin |
| Clarithromycin | Ibrutinib | Rifabutin | Zidovudine |
| Dasatinib | Imipramine | Riociguat | Zolpidem |
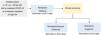
Remdesivir is also the treatment of choice for pediatric patients under the age of 12 years or weighing less than 40kg (Fig. 2). Classes of drugs other than monoclonal antibodies should be considered in patients whose condition is worsening despite remdesivir.
In conclusion, immunomodulators are a mainstay treatment for many patients in dermatology, where inflammatory and autoimmune disorders are common. Dermatologists must be familiar with the treatments available for SARS-CoV-2 infection, as some of their patients might be in a high-risk situation. We hope that this guide will help.
FundingThe authors declare that there was no funding from any entity in this research.
Conflicts of InterestThe authors declare that they have no conflicts of interest.

![Treatment algorithm for new antivirals against SARS-CoV-2 in adults (adapted from Spanish Agency for Medicines and Medical Devices [AEMPS] guidance). BAU indicates binding antibody units; Ig, immunoglobulin.](https://static.elsevier.es/multimedia/00017310/0000011400000001/v1_202301060625/S0001731022009371/v1_202301060625/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Treatment algorithm for new antivirals against SARS-CoV-2 in pediatric patients (adapted from Spanish Agency for Medicines and Medical Devices [AEMPS] guidance).](https://static.elsevier.es/multimedia/00017310/0000011400000001/v1_202301060625/S0001731022009371/v1_202301060625/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



