Sentinel lymph node biopsy is the most important tool available for node staging in patients with melanoma.
ObjectivesTo analyze sentinel lymph node detection and dissection with radio guidance from a portable gamma camera. To assess the number of complications attributable to this biopsy technique.
MethodsProspective observational study of a consecutive series of patients undergoing radioguided sentinel lymph node biopsy. We analyzed agreement between nodes detected by presurgical lymphography, those detected by the gamma camera, and those finally dissected.
ResultsA total of 29 patients (17 women [62.5%] and 12 men [37.5%]) were enrolled. The mean age was 52.6 years (range, 26-82 years). The sentinel node was dissected from all patients; secondary nodes were dissected from some. In 16 cases (55.2%), there was agreement between the number of nodes detected by lymphography, those detected by the gamma camera, and those finally dissected. The only complications observed were seromas (3.64%). No cases of wound dehiscence, infection, hematoma, or hemorrhage were observed.
ConclusionsPortable gamma-camera radio guidance may be of use in improving the detection and dissection of sentinel lymph nodes and may also reduce complications. These goals are essential in a procedure whose purpose is melanoma staging.
La biopsia selectiva del ganglio centinela representa la herramienta más importante para determinar el estatus ganglionar en el paciente con melanoma.
ObjetivosAnalizar la capacidad de detección y disección de la técnica de biopsia selectiva de ganglio centinela con la incorporación de una gammacámara portátil intraoperatoria, así como las morbilidades derivadas de la misma.
MétodosEstudio observacional sobre una serie de casos incluidos prospectivamente y de forma consecutiva de pacientes a los cuales se les realizó la técnica de biopsia selectiva de ganglio centinela radioguiada mediante gammacámara portátil. Se realizó un análisis de concordancia entre los ganglios detectados entre la linfografía prequirúrgica, la gammagrafía portátil y los ganglios disecados.
ResultadosDurante el período de estudio se incluyeron 29 pacientes diagnosticados de melanoma cutáneo. Eran 17 mujeres (62,5%) y 12 varones (37,5%) y la edad media fue de 52,6 años (rango: 26-82). Se realizó disección del ganglio centinela en el 100% de los pacientes; además se obtuvieron en algunos casos ganglios secundarios.
En el número detectado de ganglios mediante linfografía preoperatoria hubo concordancia con respecto a la gammagrafía portátil y a los ganglios disecados finalmente en 16 de los pacientes (55,2%). Respecto a las complicaciones se observaron solo seromas en un 3,64% de los casos, no presentando en ningún caso dehiscencia de la herida, infección, hematoma o hemorragia.
ConclusionesLa biopsia selectiva de ganglio centinela radioguiada mediante gammacámara portátil es una técnica que podría colaborar en mejorar la capacidad de detección y disección del ganglio centinela, así como en una disminución en la morbilidad quirúrgica derivada de la técnica, aspectos que resultan esenciales en una técnica cuyo objetivo inicial es la estadificación del paciente con melanoma.
Lymph node status is a key predictor of recurrence and survival in melanoma, and there is a direct association between prognosis and the number of affected nodes in the dissected specimen. Exhaustive examination of lymph node regions has thus become an essential part of melanoma management.1,2
Sentinel lymph node biopsy (SNB) is the most important tool available for evaluating lymph node status. Because it is a staging rather than a therapeutic procedure,3,4 maximum efforts must be made to minimize surgical morbidity. The use of portable gamma cameras has improved the effectiveness of SNB and reduced associated morbidity.5
In this study, we describe the technique employed in radioguided SNB using a gamma camera, analyze its effectiveness in terms of SN detection and dissection, and assess surgical morbidity.
Material and MethodsWe performed a prospective observational study of a series of consecutively recruited patients who underwent radioguided SNB with a gamma camera. The patients were seen at the melanoma unit of the dermatology department and the nuclear medicine department of Hospital Universitario Virgen Macarena in Seville, Spain between September 2008 and June 2010.
All patients seen at the melanoma unit during this period were consecutively recruited if they met the following inclusion criteria: presence of a primary melanoma with a Breslow thickness of 1mm or greater or of a primary melanoma with a Breslow thickness of 0.75 to 1mm in addition to ulceration, regression of over 75%, an elevated mitotic rate, or Clark level IV-V. Patients with nodal involvement or distant metastases were excluded. Staging and decisions regarding the indication of SNB were guided by the recommendations of the sixth edition (2002) of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual up to December 2009 and of the seventh edition (2009) from January 2010 onwards.1 According to the protocol in place at the melanoma unit, prior to undergoing SNB, all patients underwent computed tomography of the chest and abdomen and ultrasound evaluation of lymph node basins to rule out metastatic disease, a contraindication for SNB. As described in the protocol, patients with SNs identified by preoperative lymphoscintigraphy in the groin and axillary areas undergo SNB in the surgical unit of the dermatology department, while those with SNs detected in the head and neck region (including the parotid region) undergo SNB in the maxillofacial surgery unit. The hospital melanoma committee decides who should perform and who is responsible for these procedures.
The procedure consists of 3 stages: preoperative lymphoscintigraphy, intraoperative mapping, and SN dissection.
Preoperative LymphoscintigraphyLymphoscintigraphy is performed in the nuclear medicine department on the day before the SNB.
Four intradermal injections of 2mCi Nanocoll (or 1mCi in the case of delayed evaluation) in a volume of 0.4 mL are made around the lesion or scar (0.1 mL at each injection site). Dynamic images are obtained immediately (60 images/20 sec). In the subsequent static acquisition phase (300 seconds), different projections are obtained depending on the location of the primary lesion. Whole-body imaging is used when considered opportune. The location of nodes identified as SNs is marked on the skin with indelible ink using dots (anterior projection) and crosses (lateral projection). If an in-transit node is visualized, 0.4mCi of Nanocoll can be reinjected moments before the SNB.
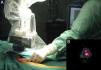
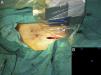
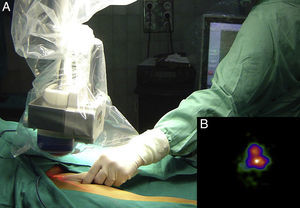
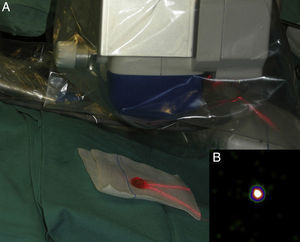
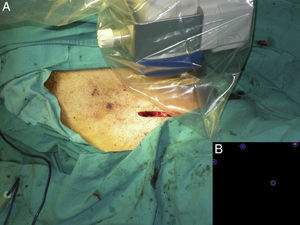
Intraoperative mapping and SN dissectionAll nodal basins and in-transit metastases identified by lymphoscintigraphy are assessed by SNB. Just before the procedure, the nuclear medicine physician uses a portable gamma camera (Sentinella Oncovision) to perform radioguided localization in the lymph node region containing the sentinel node identified on the lymphoscintigram the previous day (Fig. 1). The physician selects the exact location for incision by using a technetium probe to identify the center of the region with the highest counts. The incision is made using the cold scalpel technique. The next step is to identify and dissect the SN using the acoustic signal and uptake of vital blue dye as a guide. Before the node is removed, it is confirmed that it is indeed an SN by comparing in vivo images taken from different angles by the gamma camera to the percutaneous gammagraphic image taken in the immediate preoperative phase. Following dissection, images are taken of both the excised node (Fig. 2) and the surgical field to check for hot spots corresponding to secondary SNs, which, if identified, are also removed. The nuclear medicine physician confirms absence of activity in the surgical bed using the gamma camera (Fig. 3). Appropriately labeled formalin-fixed SNs are sent to the pathologist for histological examination with hematoxylin-eosin and an immunohistochemical study with HMB-45, S-100, and Melan-A.
We analyzed our results by calculating the percentage agreement between the different procedures (in terms of the number of nodes identified and dissected) and performed a descriptive analysis of procedure-related complications using Excel for Mac 2010.
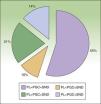
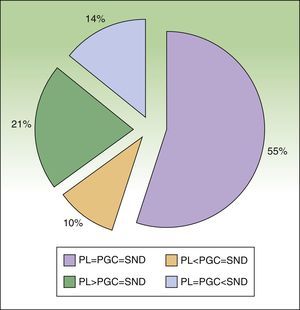
ResultsTwenty-nine patients with cutaneous melanoma and indications for SNB (≥stage IB with no clinical or radiological evidence of spread to nearby lymph nodes [N0] and no distant metastasis [M0]) were included in the study between September 2008 and June 2010. All the patients underwent radioguided SNB with a portable Sentinella gamma camera. There were 17 women (62.5%) and 12 men (37.5%) with a mean age of 52.6 years (range, 26-82 years). The lower limbs were the most common melanoma site (43.7%), followed by the trunk (28.1%) and the upper limbs (21.8%). The mean Breslow thickness was 1.90mm (range, 0.79-9.00mm). Eight patients (27.5%), had thin melanomas (≤1mm), 16 (55.2%) had intermediate-thickness melanomas (1.01-4.00mm), and 6 (17.2%) had thick melanomas (>4mm). The histologic subtypes were superficial spreading melanomas in 50% of cases, nodular melanomas in 34.3%, and acral lentiginous melanomas in 16.6%. Ulceration and a mitotic rate of at least 1 mitosis/mm2 were each observed in 5 cases (17.2%). Six patients (20.7%) had signs of regression. There was no variation between presurgical and postsurgical staging, as all nodes removed were negative for metastasis. Nodal involvement was detected in just 1 patient during the first 3 years of follow-up. The lymphoscintigraphy showed uptake in a single drainage basin in 25 patients (86.2%) and in 2 basins in 4 (13.8%). SN dissection was achieved in 100% of cases, and in some cases, secondary SNs were also identified and removed (Table 1). The number of nodes identified by preoperative lymphoscintigraphy and portable gamma camera detection was identical in 16 patients (55.2%). In 9 patients (31%), the number of SNs detected using the gamma camera was the same as the number of SNs dissected but different to that of SNs observed by lymphoscintigraphy. This preoperative evaluation identified more nodes in 6 of the cases (21%) and fewer nodes in the other 3 cases (10%) (Fig. 4). Finally, the number of SNs excised was higher than that detected by either lymphoscintigraphy or gamma camera in 4 patients (13.7%). The only complications observed were seromas (3.6% of cases), and these all resolved spontaneously, with no need for aspiration. There were no cases of wound dehiscence, infection, hematoma, or hemorrhage.
Characteristics of Patients and Melanomas With Location of Drainage Basins, Number of Sentinel Nodes Identified by Preoperative Lymphoscintigraphy (PL) and Portable Gamma Camera Detection (PGC), and Number of Nodes Dissected (SND).
| Age, y | Melanoma Site | Breslow Thickness, mm | Drainage Basin | PL | PGC | SND |
|---|---|---|---|---|---|---|
| 51 | Right leg | 2 | Inguinal (ipsilateral) | 1 | 1 | 1 |
| 75 | Right leg | 1.5 | Inguinal (ipsilateral) | 4 | 1 | 1 |
| 58 | Dorsal midline | 4.5 | Axillary (bilateral) | 3 | 3 | 3 |
| 71 | Dorsal midline | 1 | Axillary (ipsilateral) | 3 | 3 | 3 |
| 55 | Dorsum of right foot | 3.2 | Inguinal (ipsilateral) | 4 | 4 | 4 |
| 51 | Right hemithorax | 9 | Axillary (ipsilateral) | 1 | 1 | 2 |
| 64 | Right anterior thorax | 5 | Axillary (ipsilateral) | 2 | 1 | 1 |
| 47 | Dorsal midline | 1.9 | Axillary (bilateral) | 2 | 2 | 2 |
| 66 | Right dorsolumbar region | 2 | Axillary (ipsilateral) | 4 | 2 | 2 |
| 67 | Left periumbilical region | 0.85 | Inguinal (ipsilateral) | 1 | 2 | 2 |
| 56 | Left popliteal fossa | 1 | Inguinal (ipsilateral) | 4 | 2 | 2 |
| 48 | Right gluteus | 1.1 | Inguinal (ipsilateral) | 2 | 2 | 2 |
| 53 | Right forearm | 2 | Axillary (ipsilateral) | 1 | 1 | 1 |
| 40 | Right wrist | 2.1 | Axillary (ipsilateral) | 1 | 1 | 1 |
| 48 | Right supraclavicular region | 0.9 | Supraclavicular (ipsilateral) | 2 | 2 | 2 |
| 47 | Right leg | 1.5 | Inguinal (ipsilateral) | 5 | 1 | 1 |
| 25 | Left clavicle | 1.3 | Axillary and supraclavicular (ipsilateral) | 4 | 6 | 6 |
| 38 | Right thigh | 1.2 | Inguinal (ipsilateral) | 3 | 3 | 3 |
| 25 | Left supraclavicular region | 0.9 | Axillary (ipsilateral) | 1 | 4 | 4 |
| 52 | Right periscapular region | 0.7 | Axillary (ipsilateral) | 4 | 4 | 4 |
| 50 | Right leg | 0.8 | Inguinal (ipsilateral) | 1 | 2 | 3 |
| 63 | Right leg | 1.5 | Inguinal (ipsilateral) | 5 | 3 | 3 |
| 35 | Left thigh | 1.8 | Inguinal (ipsilateral) | 2 | 2 | 2 |
| 64 | Left arm | 1.1 | Axillary (ipsilateral) | 1 | 1 | 1 |
| 45 | Left scapular region | 1 | Axillary (ipsilateral) | 3 | 1 | 2 |
| 59 | Left dorsolumbar region | 1.5 | Axillary (ipsilateral) and scapular | 4 | 4 | 6 |
| 67 | Dorsum of left foot | 0.93 | Inguinal (ipsilateral) | 2 | 2 | 2 |
| 65 | Right forearm | 2 | Axillary (ipsilateral) | 2 | 2 | 2 |
| 40 | Left shoulder | 0.79 | Axillary (ipsilateral) | 1 | 2 | 2 |
Percentage agreement between preoperative lymphoscintigraphy (PL), portable gamma camera detection (PGC), and sentinel node dissection (SND) in terms of number of nodes identified and dissected.
PL=PGC=SND: full agreement; PL<PGC=SND: fewer nodes detected by preoperative lymphoscintigraphy than those detected by gamma camera and dissected; PL>PGC=SND: more nodes detected by preoperative lymphoscintigraphy than those detected by gamma camera and dissected; PL=PGC<SND: more nodes dissected than those detected by lymphoscintigraphy or gamma camera.
The therapeutic and prognostic value of SNB has been much discussed in the past decade. The results of the Multicenter Selective Lymphadenectomy Trial (MSLT) by Morton et al.2,3 established the benefits of and indications for SNB in a large prospective study of patients with melanomas with a Breslow thickness of between 1.20 and 3.50mm. This randomized, controlled trial provided the strongest evidence up to then on the value of SNB as a staging tool and a technique for the regional control of disease in patients with moderate risk. This evidence was further strengthened in a subsequent study of 1270 patients with a 10-year follow-up period.5 If we thus accept that SNB is a staging rather than a therapeutic tool,3–5 all reasonable efforts should be made to ensure maximum SN dissection and minimal surgical morbidity.
With the double aim of improving the sensitivity of SNB and reducing associated morbidity, the dermatology and nuclear medicine departments of Hospital Universitario Virgen Macarena started to use a Sentinella mobile gamma camera during the procedure in the belief that it would result in improved SN identification with few surgical complications.
Prior to the introduction of portable gamma cameras, the addition of gamma probe detection to intraoperative mapping with vital blue dye6 increased the sensitivity of SNB in the identification and dissection of SNs and also reduced morbidity.7–9 In a retrospective study of 430 patients who underwent SNB combining the intraoperative use of vital blue dye and a gamma probe, Landi et al.7 reported a 99.5% excision success rate. They were unable to remove SNs in 2 patients; one had a node in the cervical area and the other had a node in the axillary area. Similar success rates have been reported in other series, with Leong et al.8 and Kaptejin et al.9 reporting respective dissection rates of 98% (163 patients) and 99% (110 patients). The results from our series show that the use of a portable gamma camera resulted in the excision of at least 1 SN in 100% of cases. The camera also identified occult SNs in 10% of cases, i.e. SNs that had not been detected by preoperative lymphoscintigraphy due to overlapping with the predominant SN. Our findings show agreement between the 3 procedures—preoperative lymphoscintigraphy, intraoperative radioguided detection with a gamma camera, and dissection—in over half (55.2%) of the cases analyzed. In other words, localization of the SN would be much more certain in this subgroup of patients. Fewer nodes were excised than those identified by lymphoscintigraphy in 21% of patients, all of whom had groin involvement. Lymph nodes in this area are iliac nodes, and therefore have a deep location. Because SNB is considered a staging not a therapeutic procedure, it is not indicated for the removal of iliac nodes given the considerable morbidity associated with this location. Finally, more nodes were excised than those detected either preoperatively or intraoperatively using the portable gamma camera in 4 patients (13.7%). This typically occurs when clusters of nodes appear as a single node in the imaging studies. In our experience, gamma probes have certain limitations related to SN depth, i.e., preincisional uptake by nodes is more complicated in patients with thick adipose tissue, and furthermore the correlation between the acoustic signal emitted and the number of counts per minute is variable. In other words, the definition of what can be considered an SN using this method is variable.10 Prior to the use of portable gamma cameras, SNB was guided by information provided by static images taken after the injection of technetium nanocolloid on the day before the procedure and immediately before the procedure; this information was used to mark the locations corresponding to the anterior and lateral projections of the presumed SN on the patient's skin.7 The location of the surgical incision site was determined using gamma probes, but this procedure can be complicated by the position of the patient, the thickness of the subcutaneous tissue, the depth of the SN, and background activity from the primary tumor.10
The intraoperative use of portable gamma cameras has essentially improved SN identification and dissection rates,11 and these cameras are now widely used in multiple surgical procedures in the field of oncology.12–18 The camera produces a real map of the possible location of the SN, showing the exact site of the surgical incision, meaning that shorter incisions are needed. Ex vivo imaging of the excised node is then used to confirm whether or not it is an SN. Finally, the camera is used to confirm, intraoperatively, that the surgical field is clean, i.e., it confirms disappearance of the uptake detected prior to dissection.11 Before the introduction of gamma cameras, the surgical field was checked postdissection with a gamma probe, but it can be difficult to differentiate between background counts and counts from occult secondary SNs following high focal uptake by a gammagraphically predominant SN. Intraabdominal location and activity from the area of the primary tumor can also make it difficult to correctly identify SNs.10
None of the patients in our series experienced hematoma, hemorrhage, or wound dehiscence, and only 3.64% developed seromas, which all resolved spontaneously, without aspiration. By way of comparison, a complication rate of 4.2% was reported in the largest series to date of patients to undergo SNB with intraoperative vital blue mapping and gamma probe detection.7
The main limitation of our study is the absence of cases involving the head and neck region.
To conclude, SNB is the standard tool for determining lymph node status, the most important prognostic factor in the current melanoma staging system. The procedure, however, has not yet shown any benefits in terms of disease-free or overall survival in melanoma patients without nodal involvement. The direct visualization offered by radioguided SNB with a portable gamma camera could therefore help to improve the sensitivity of the technique and reduce surgical morbidity, 2 essential aspects in a procedure whose purpose is, at least to date, to offer the most complete staging information possible in patients with melanoma. This study shows that radioguided SNB with direct visualization can help to achieve SN identification and excision rates of 100% and may also reduce morbidity compared with previous techniques. We believe that this reduction in complications is essential in a surgical procedure that still has a largely nontherapeutic use.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Peral Rubio F, de La Riva P, Moreno-Ramírez D, Ferrándiz-Pulido L. Biopsia selectiva del ganglio centinela radioguiada mediante gammacámara portátil. Estudio observacional sobre serie de casos incluidos prospectivamente de forma consecutiva. Actas Dermosifiliogr. 2015;106:408–414.