Topical corticosteroids are used to treat inflammation and relieve itching in atopic dermatitis, but their use is limited by adverse reactions.
ObjectivesThe main aim of this study was to investigate whether daily treatment with Polypodium leucotomos extract would reduce the use of topical corticosteroids in children and adolescents with atopic dermatitis. We also analyzed oral antihistamine use and changes in disease severity.
Patients and methodsWe performed a phase IV randomized, double-blind, placebo-controlled, multicenter trial involving 105 patients aged between 2 and 17 years who were receiving topical corticosteroids to treat moderate atopic dermatitis. The patients were randomized to receive, in addition to their standard treatment, Polypodium leucotomos extract or placebo (both in capsule form) for 6 months. The percentage of days on which topical corticosteroids and other atopic dermatitis treatments were used was calculated.
ResultsUse of Polypodium leucotomos extract did not significantly reduce the mean (SD) percentage of days on which topical corticosteroids were used (11% [12%] vs 12% [11%] for placebo). A significant reduction was, however, observed for oral histamine use (median percentage of days, 4.5% in the Polypodium leucotomos group and 13.6% in the placebo group [P=.038]). The percentage of patients who used oral antihistamines was also lower in the Polypodium leucotomos group.
ConclusionLong-term treatment with Polypodium leucotomos extract has benefits for children and adolescents with atopic dermatitis who require pharmacologic treatment to reduce inflammation and relieve itching.
Para el control de la lesión inflamatoria de la dermatitis atópica, y secundariamente del prurito, se utilizan corticosteroides tópicos, pero su empleo está limitado por las reacciones adversas.
ObjetivosEl objetivo primario del estudio fue evaluar si el tratamiento diario con extracto de Polypodium leucotomos permitiría reducir el uso de corticosteroides tópicos en niños y adolescentes con dermatitis atópica. Secundariamente se valoró el consumo de antihistamínicos orales, así como la evolución de la enfermedad.
Pacientes y métodosSe ha realizado un ensayo clínico en fase iv, multicéntrico, aleatorizado y doble ciego de extracto de Polypodium leucotomos, controlado con placebo, en 105 pacientes de 2 a 17 años de edad, con dermatitis atópica de intensidad moderada e indicación de corticosteroides tópicos. Los pacientes recibieron durante 6 meses extracto de Polypodium leucotomos o placebo por vía oral añadidos al protocolo terapéutico habitual, y se calculó el porcentaje de días en que se utilizaban corticosteroides tópicos u otros tratamientos para la dermatitis atópica.
ResultadosEl extracto de Polypodium leucotomos redujo de modo no significativo el uso de corticosteroides tópicos (11±12% de días), comparado con placebo (12±11%). El porcentaje de días en los que los pacientes requirieron antihistamínicos orales fue significativamente menor con extracto de Polypodium leucotomos (mediana de 4,5% días) que con placebo (13,6%) (p=0,038). También se redujo el porcentaje de pacientes que tomaron antihistamínicos orales.
ConclusionesEl tratamiento prolongado con extracto de Polypodium leucotomos aporta beneficios relevantes para los pacientes en edad pediátrica con dermatitis atópica que precisan tratamiento farmacológico para controlar la lesión inflamatoria y reducir el prurito.
Atopic dermatitis is a chronic inflammatory skin disease characterized by intense pruritus, dry skin, inflammation, and exudate. It is often associated with asthma, allergic rhinitis, food allergy, and recurrent secondary infection. Symptoms usually first appear in childhood, and approximately 50% of cases are diagnosed in the first year of life.1–3 In developed countries, atopic dermatitis affects approximately 10% to 15% of children under the age of 5 years at some time, and childhood prevalence is in the range of 10% to 30%.2,4 While the disease tends to be mild, it can have considerable repercussions on the quality of life of both children and adults; its economic and family impact may be higher than that of psoriasis and similar to that of other serious diseases such as early-onset diabetes mellitus.1,2,4,5
There is general consensus concerning the treatment of atopic dermatitis in Spain.1,2,6–8 The primary goals of treatment are to relieve itching, control the inflammation, and prevent relapse. H1 antihistamines are the treatment of choice for relieving itching. Topical corticosteroids administered daily and calcineurin inhibitors administered in different regimens are used to control inflammation during flares or acute exacerbarations.1,2,6,7 Hygiene and dietary measures are prescribed to prevent relapse; examples are avoidance of contact with specific triggers and allergens (including food), as well as with nonspecific triggers such as excessive heat and humidity.
The use of topical corticosteroids may be limited by adverse reactions at the application site.1,2,6–9 More serious systemic adverse effects may also occur but they are much rarer.6,10 Approximately 70% to 80% of parents or carers of children with atopic dermatitis are concerned about the adverse effects of topical corticosteroids and this leads to reduced treatment adherence; approximately 25% of parents and carers have reported that they avoid topical corticosteroids because of anxiety.6 New drugs, such as topical calcineurin inhibitors, have emerged as alternatives to corticosteroids to treat inflammation, but there are still safety concerns about the long-term use of these products.11
Polypodium leucotomos (PL) extract is a drug product licensed for the treatment of atopic dermatitis in adults and children of any age; it has been available in Spain for years. The aim of this clinical trial was to determine whether daily administration of PL extract in addition to standard treatment might reduce the use of topical corticosteroids and antihistamines in patients with moderate atopic dermatitis aged between 2 and 17 years who require these drugs to treat flares.
MethodsPatients and RandomizationWe performed a randomized, double-blind, placebo-controlled, multicenter phase IV study of oral PL extract (Anapsos) administered over 6 months. The study was performed in 9 hospitals in the Autonomous Community of Valencia, Spain (see the members of the Anapsos in Atopic Dermatitis Work Group in Appendix 1). Patients were randomized to receive PL extract or placebo at a ratio of 1:1. Randomization, with stratification per center, was performed electronically prior to initiation of the study. The study protocol (EudraCT 2008-006422-33) complied with the principles of the Declaration of Helsinki (2008) and was approved by the relevant clinical research ethics committees and the Spanish Agency for Medicines and Health Products. Parents or legal guardians and children older than 12 years gave their written consent to being included in the study.
Inclusion criteria were an age of between 2 and 17 years (inclusive) and a diagnosis of atopic dermatitis according to the Hanifin and Rajka criteria.12 Disease severity was evaluated using the Scoring Atopic Dermatitis (SCORAD) index.13 To be included in the study, patients had to have a SCORAD score of 20 to 40 (inclusive) and require or already be using topic corticosteroids to treat atopic dermatitis flares.
Exclusion CriteriaThe main exclusion criteria were pregnancy or breastfeeding; current treatment with phototherapy for atopic dermatitis; treatment with PL extract, systemic corticosteroids, or other systemic or cytostatic immunosuppressants in the previous 2 months; treatment with systemic antimicrobials in the 4 days before the start of the study; fever; severe allergic disease; immunodeficiency disorders or cancer; contraindication for the use of any of the study drugs according to the summaries of product characteristics; known allergy to parabens; concomitant skin disease that could make it difficult to evaluate the atopic dermatitis or that required long-term use of topical corticosteroids; and participation in drug research studies in the 3 months before the start of the study. Women of childbearing age were required to use an effective birth control method if they were or became sexually active during the trial. The criteria for withdrawal was use of corticosteroids for more than 5 days in patients under the age of 10 years and for more than 7 days in patients aged between 10 and 17 years.
TreatmentThe patients received PL extract capsules (Anapsos 120 mg manufactured by Especialidades Farmacéuticas Centrum S.A) or similar placebo capsules produced by the same laboratory. Based on the recommendations of previous studies,14,15 the patients were divided into 3 treatment groups according to age (< 6 years, 6-12 years, and >12 years). Children aged less than 6 years received 2 capsules in a single dose at night (240 mg/day of PL extract or placebo); children aged 6 to 12 years received 3 capsules in 2 doses: 1 in the morning and 2 at night (360 mg/day); and children over 12 years received 4 capsules in 2 doses: 2 in the morning and 2 at night (480 mg/day).
Methylprednisolone aceponate 0.1% in emulsion was applied in a thin layer on affected areas to treat flares. Dry skin was treated symptomatically with moisturizing cream after showering or bathing. Patients who required systemic corticosteroids were administered deflazacort at a dosage of 0.25 to 1.5 mg/kg/d. Desloratadine was used to treat pruritus as it comes in tablet and liquid form and dosage could be adjusted to the ages of all the patients in the study. It is administered once daily and because it is a low-sedating antihistamine, it exerts a minimum effect on the sleep loss component of the SCORAD index. The following dosages were used: 2.5 mL of syrup (1.25 mg of desloratadine) once a day in children aged 2 to 5 years; 5 mL (2.5 mg) once a day in children aged 6 to 11 years; and 1 tablet (5 mg) once a day in children aged 12 or over.
Methods and Study VariablesThirteen visits, to take place every 2 weeks after the start of treatment, were scheduled once patients had attended the inclusion visit and been assigned to treatment. To collect data on the use of medication, number of flares, and adverse events, the parents or carers were given a diary in which they were asked to note down in detail and objectively any information on the use of medication and the appearance of new health problems. Scheduled blood and urine tests were performed before and after the study. A pregnancy test was performed in patients of child-bearing age before and after the study and once a month for the duration of the study.
The primary outcome measure was the percentage of days on which topical corticosteroids were used in the 6 months in which patients received PL extract or placebo in addition to their standard treatment. Prespecified secondary outcome measures (based on systematically collected data) were changes in the SCORAD index and investigator's global assessment (IGA) 1, 2, 3, 4, 5, and 6 months after the start of treatment; time to first flare (time from randomization to appearance of a new flare); changes in the incidence of flares (based on mean number of flares reported for the 2 months before the baseline visit); and adverse events. A flare was defined as an exacerbation that could be quantified, following evaluation of the patient's diary, physical examination, and an interview with the patient or tutor, with an IGA score of 4 or more, or that required the use of topical corticosteroids for at least 3 days. A course of corticosteroids was defined as the use of corticosteroids according to the prescribed regimen for an interrupted period, regardless of the number of days.
Statistical AnalysisContinuous quantitative variables were reported as means (SD), categorical variables as numbers and percentages, and time to first flare as a median and range. All the data were analyzed using intention-to-treat analysis (all patients included in the study) and per-protocol analysis (all those who completed follow-up). The last observation carried forward method was used for patients who did not complete follow-up. The percentage of days on which topical corticosteroids or antihistamines were used was calculated as the number of days on which treatment was used divided by the total number of days the study lasted. The PL extract and placebo groups were compared using the Wilcoxon test, with statistical significance set at 5%. Bonferroni adjustment was used for multiple comparisons. Sample size was calculated using data from previous studies of adults with a similar diagnosis and moderate disease in which the placebo group used topical corticosteroids for a mean of 37.2% (34.6%) of days (6-month study period).16,17 Assuming thus a 60% reduction in the days topical corticosteroids would be used in the group treated with PL extract and an estimated loss to follow-up of 20%, we calculated that we would need 53 patients per group to detect statistically significant differences for a power of 80%.
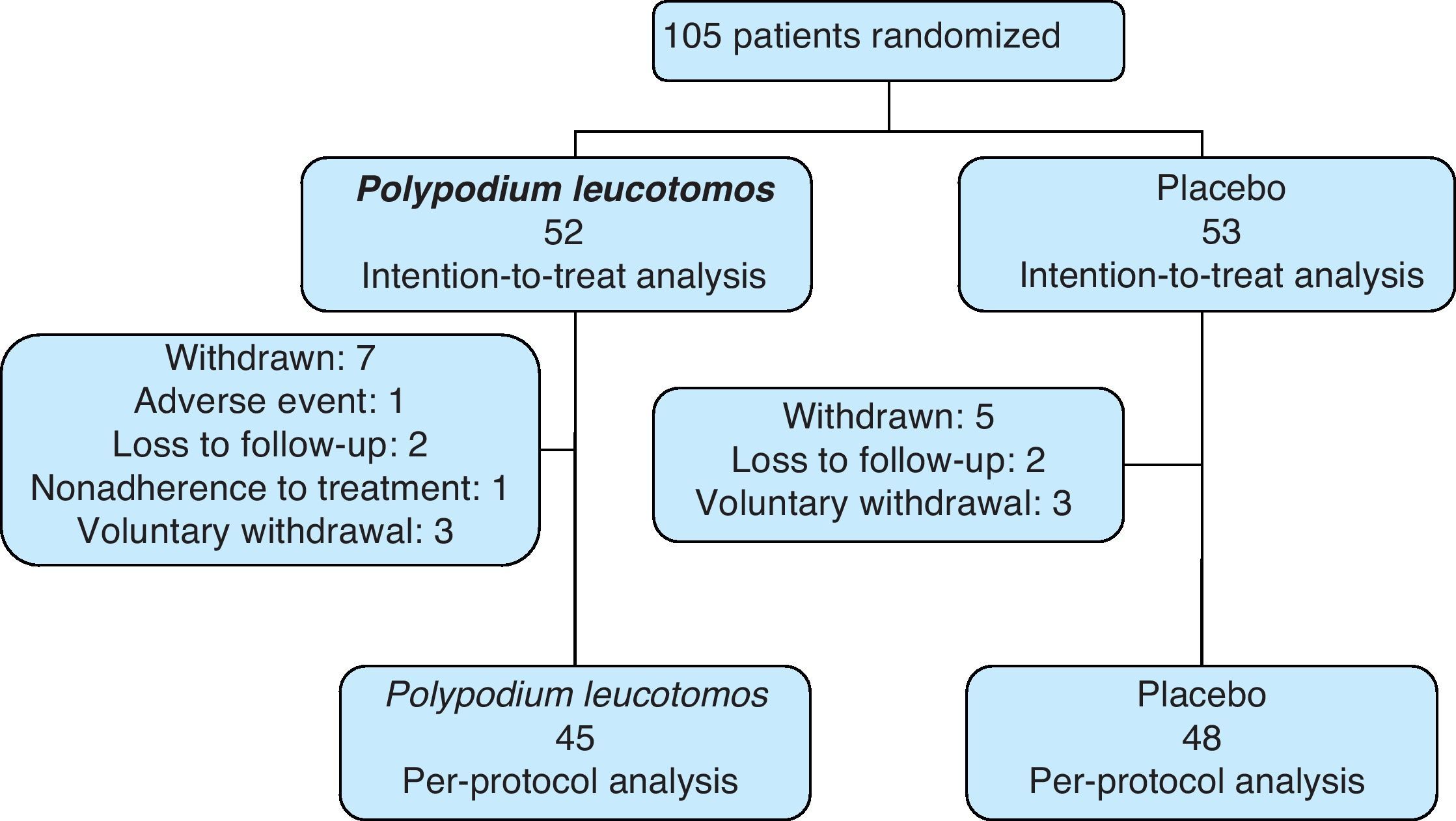
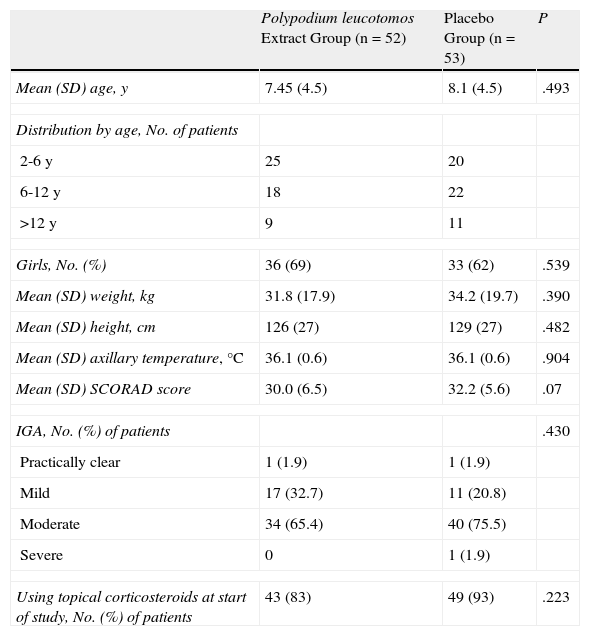
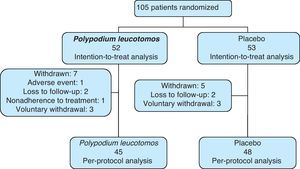
ResultsStudy ParticipantsIn total, 105 patients (69 girls) were included in the study. Ninety-three patients (89%) completed the 6 months of treatment and the number of withdrawals was similar in the PL extract group and the placebo group. Fifty-two patients were assigned to the PL extract group and 53 to the placebo group (Fig. 1). The mean SCORAD score was 31 (95% CI, 30-32). The IGA was classified as mild to moderate in 97% of cases. The mean age of the patients was 8 (4) years (range, 2-17 years), the mean weight was 33.2 (18.8) kg, and the mean axillary temperature was 36.1 (0.6) °C. All the patients met the Hanifin and Rajka diagnostic criteria and most of them met the 4 major criteria. Eighty-eight percent of patients were using topical corticosteroids at the time of the baseline visit. There were no significant differences in demographic characteristics, clinical variables related to atopic dermatitis, or clinical course between the patients in the PL extract group and those in the placebo group (Table 1).
Baseline Characteristics of Study Participants.
| Polypodium leucotomos Extract Group (n=52) | Placebo Group (n=53) | P | |
| Mean (SD) age, y | 7.45 (4.5) | 8.1 (4.5) | .493 |
| Distribution by age, No. of patients | |||
| 2-6 y | 25 | 20 | |
| 6-12 y | 18 | 22 | |
| >12 y | 9 | 11 | |
| Girls, No. (%) | 36 (69) | 33 (62) | .539 |
| Mean (SD) weight, kg | 31.8 (17.9) | 34.2 (19.7) | .390 |
| Mean (SD) height, cm | 126 (27) | 129 (27) | .482 |
| Mean (SD) axillary temperature, °C | 36.1 (0.6) | 36.1 (0.6) | .904 |
| Mean (SD) SCORAD score | 30.0 (6.5) | 32.2 (5.6) | .07 |
| IGA, No. (%) of patients | .430 | ||
| Practically clear | 1 (1.9) | 1 (1.9) | |
| Mild | 17 (32.7) | 11 (20.8) | |
| Moderate | 34 (65.4) | 40 (75.5) | |
| Severe | 0 | 1 (1.9) | |
| Using topical corticosteroids at start of study, No. (%) of patients | 43 (83) | 49 (93) | .223 |
Abbreviations: IGA, investigator's general assessment; SCORAD, Scoring Atopic Dermatitis index.
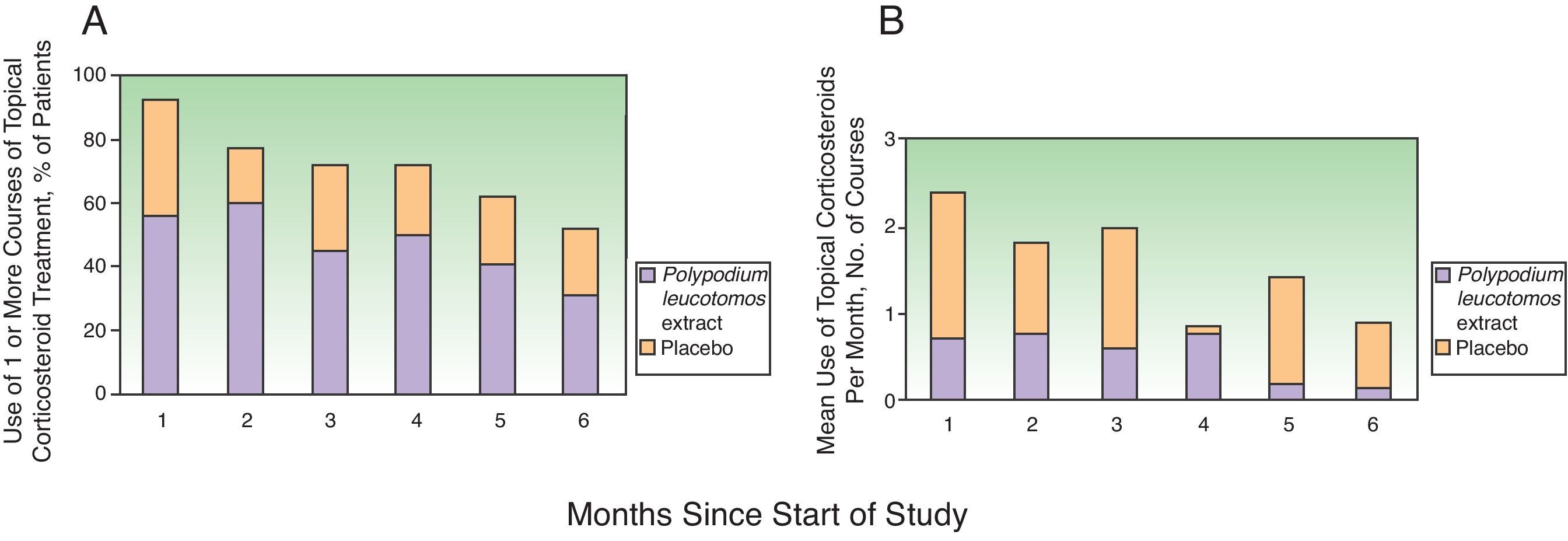
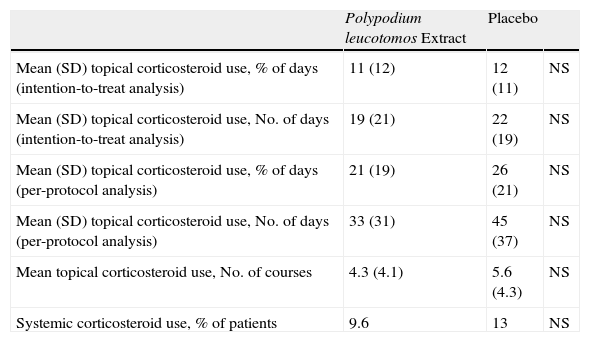
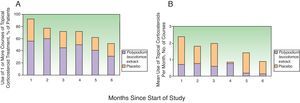
Topical corticosteroids were used on 11% (12%) of the days in the study period by the patients in the PL extract group and on 12% (11%) of the days by those in the placebo group (P=.2) (Table 2). Table 2 also shows a lower use of topical corticosteroids in the PL extract group for all the other variables analyzed but the differences with the placebo group were not significant in any of the cases. Fig. 2, in turn, shows a lower percentage of patients requiring topical corticosteroids and a lower mean number of courses of treatment in the PL extract group over the course of the study, but again, the differences with the placebo group were not statistically significant.
Corticosteroid Use During the Study.
| Polypodium leucotomos Extract | Placebo | ||
| Mean (SD) topical corticosteroid use, % of days (intention-to-treat analysis) | 11 (12) | 12 (11) | NS |
| Mean (SD) topical corticosteroid use, No. of days (intention-to-treat analysis) | 19 (21) | 22 (19) | NS |
| Mean (SD) topical corticosteroid use, % of days (per-protocol analysis) | 21 (19) | 26 (21) | NS |
| Mean (SD) topical corticosteroid use, No. of days (per-protocol analysis) | 33 (31) | 45 (37) | NS |
| Mean topical corticosteroid use, No. of courses | 4.3 (4.1) | 5.6 (4.3) | NS |
| Systemic corticosteroid use, % of patients | 9.6 | 13 | NS |
Abbreviation: NS, nonsignificant difference.
Fig. 2 also shows a progressive reduction in topical corticosteroid use over the 6 months in both groups. In the placebo group, the changes with respect to the previous month were not significant for any of the months analyzed. In the PL group, however, there was a relevant and significant reduction in the second month compared to the first (median of 13.8 days in the first month vs 7.2 days in the second, P=.012) and in the fifth month compared to the fourth (median of 3 days in the fourth month vs 6 days in the fifth, P=.012).
On comparing the use of topical steroids each month with that recorded in the first month (by percentage of days), a reduction of 36% was observed in the fifth month for the PL extract group; the corresponding reduction in the placebo group was 9% (P=.02).
No significant variations in results were seen when the data were adjusted for season.
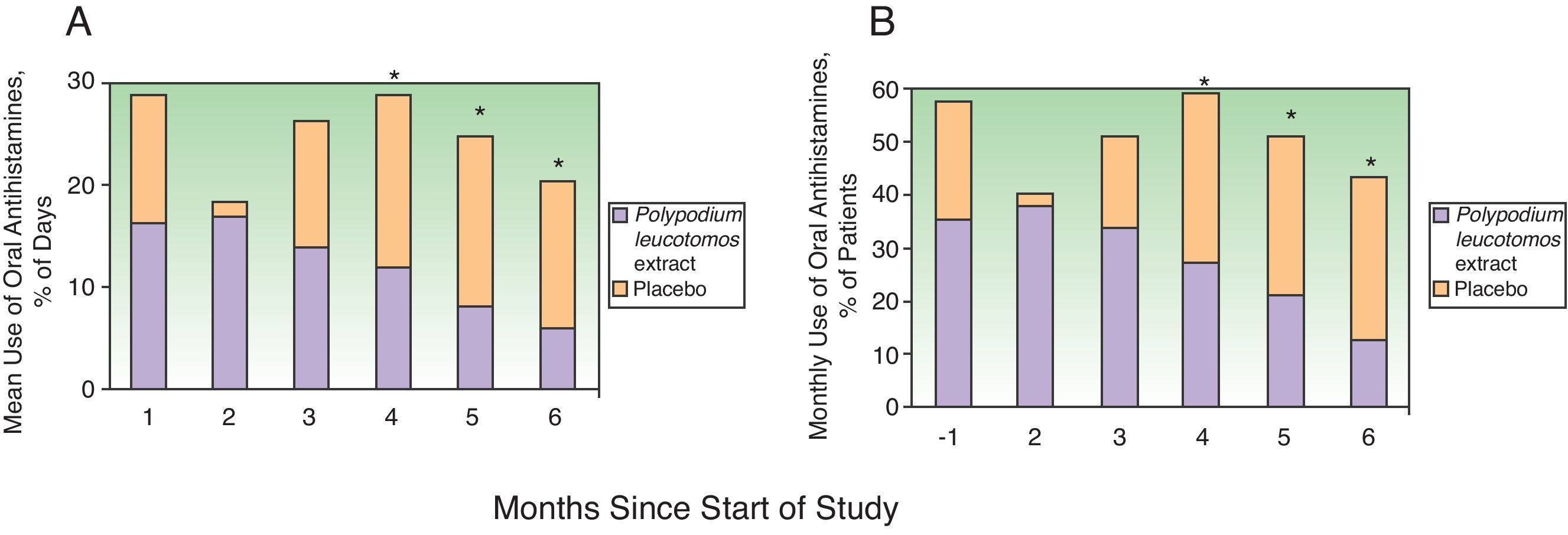
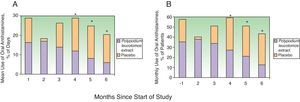
Efficacy in Terms of Antihistamine UseAntihistamine and systemic corticosteroid use was evaluated using the same approach as that described for topical corticosteroids. The percentage of days on which the patients took desloratadine over the 6 months was significantly lower in the PL extract group (mean, 14% [19%]; median, 4.5% [range, 0%-66.5%]) than in the placebo group (mean, 21% [22%]; median, 13.6% [range, 0%-86.4% (P=.038). On analyzing the percentage of days on which desloratadine was used by month, a nonsignificant reduction was seen in the first month and a significant reduction in months 4, 5, and 6 (Fig. 3A). The overall proportion of patients who required oral antihistamines was also lower in the PL extract group than in the placebo group. This difference was apparent after the first month of treatment but it became statistically significant after the third month (fourth, fifth, and sixth months) (Fig. 3B).
Use of oral antihistamines during the 6 months of the study. A, Mean percentage of days on which the patients in the Polypodium leucotomos extract and the placebo group took oral antihistamines. B, Percentage of patients who took oral antihistamines every month. * P<.05 on comparing data from the same month between groups.
No significant between-group differences were observed for the use of systemic corticosteroids (9.6% in the PL extract group vs 13% in the placebo group).
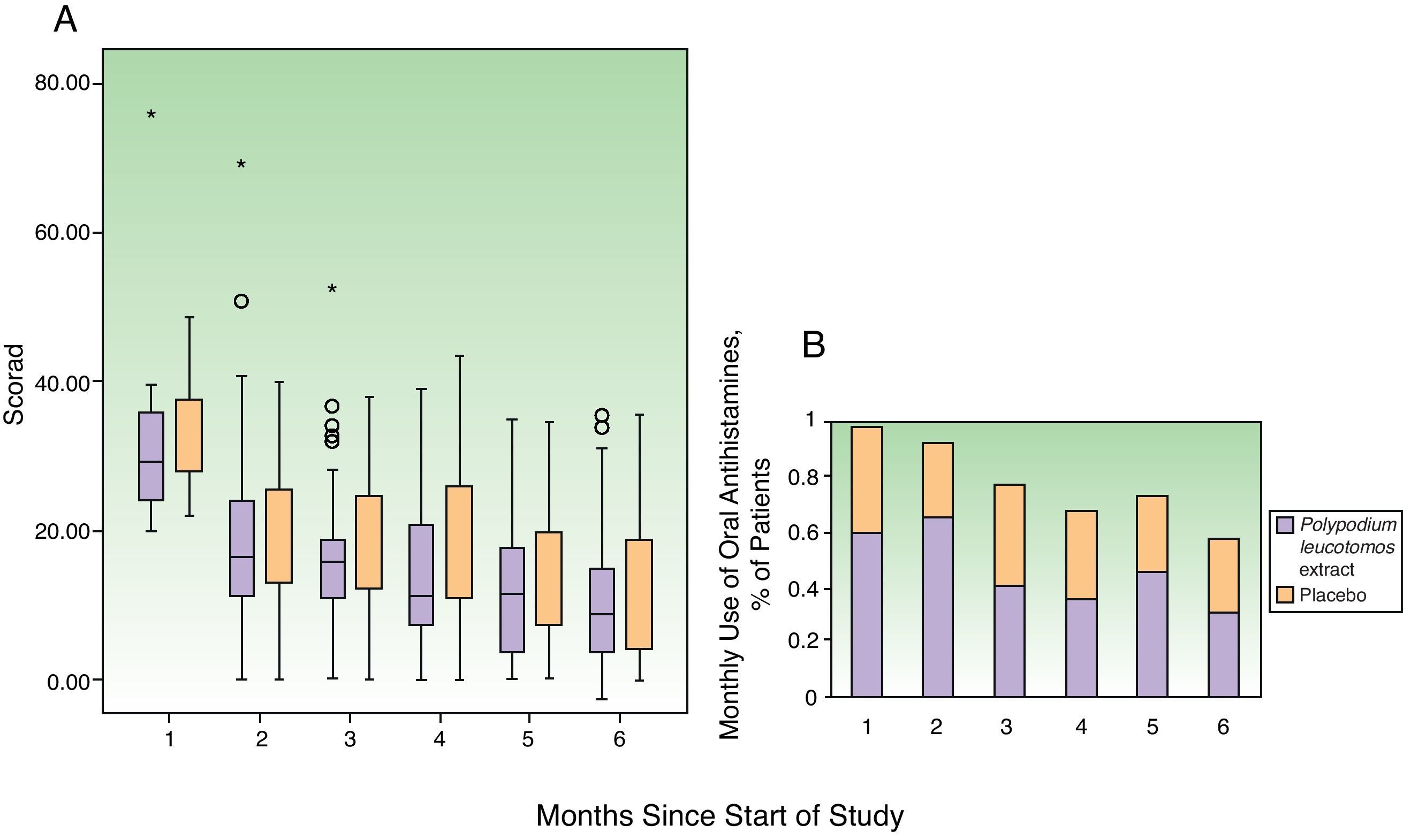
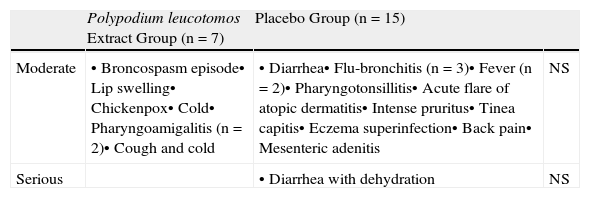
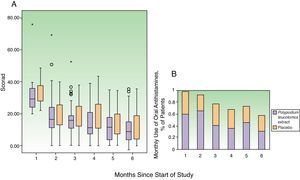
Clinical CourseFig. 4 shows the changes in the SCORAD index and the number of flares each month. The changes in the overall SCORAD index and the 3 subscores (extent, intensity, and pruritus and sleep loss) were similar in both groups. Fewer flares (total number of flares and flares recorded at each visit) were reported in the PL extract group but the differences with the placebo group were slight and statistically insignificant in all cases. There were also no significant differences between the groups in the time to the first flare (median of 0.5 months in both groups) or in the progressive reduction in disease severity assessed using the IGA. In total, 463 adverse events were recorded over the study period: 218 in the PL extract group and 223 in the placebo group. In both groups, most of the events were classified as mild. Moderate adverse events were more common in the placebo group (14 patients) than in the PL extract group (7 patients) (Table 3). Only 1 patient, receiving placebo, experienced a severe adverse event (diarrhea with dehydration).
Moderate to Severe Adverse Events During Study.
| Polypodium leucotomos Extract Group (n=7) | Placebo Group (n=15) | ||
| Moderate | • Broncospasm episode• Lip swelling• Chickenpox• Cold• Pharyngoamigalitis (n=2)• Cough and cold | • Diarrhea• Flu-bronchitis (n=3)• Fever (n=2)• Pharyngotonsillitis• Acute flare of atopic dermatitis• Intense pruritus• Tinea capitis• Eczema superinfection• Back pain• Mesenteric adenitis | NS |
| Serious | • Diarrhea with dehydration | NS |
Abbreviation: NS, nonsignificant difference.
PL extract is a drug product that increases the proportion of suppressor CD8+ lymphocytes18 and modifies cytokine release by inhibiting the proliferation of monocytes and interleukin 1ß, thereby modifying the production of cytokines by T cells, increasing CD4+ and CD3−CD16+CD56+ cells,19,20 and stimulating the proliferation and activation of suppressor T cells. It also reduces the effect of CD11, CD18, and CD62-L adhesion molecules.21 PL extract is effective in different inflammatory22 and atopic23 diseases, in particular, atopic dermatitis.15,24,25 Our results show that long-term treatment with PL extract is beneficial in pediatric patients with moderate atopic dermatitis (SCORAD score of 20-40) that requires treatment with topical corticosteroids and antihistamines. The most relevant effect of PL extract in our series was a reduction in antihistamine use measured by both the percentage of patients who used desloratadine and the percentage of days on which this drug was used. The clinical improvements noted in the PL extract group, reflected by changes in the SCORAD index, IGA, and number of flares, were similar to those seen in the placebo group, but they were achieved with lower corticosteroid and antihistamine usage. This indicates that the long-term administration of PL extract in addition to standard first-line treatment has both a beneficial effect in the treatment of atopic dermatitis and potential clinical relevance.
The main objective of the study was to investigate whether the administration of PL extract would lead to a reduction in the use of topical corticosteroids, measured by the percentage of days on which these drugs were required. While the daily administration of this drug product over 6 months did reduce the number and percentage of days on which topical corticosteroids, in addition to the percentage of patients who required systemic corticosteroids, the differences with the placebo group were not statistically significant.
In other studies with a similar design to ours, corticosteroid use varied less over the course of treatment, and levels at the end of the 6 months were similar to initial levels.16,17 There are, however, certain methodological differences between these studies and ours that may be relevant. First, unlike in our study, the use of antihistamines was not permitted in patients who could not guarantee adherence to a stable treatment regimen for the duration of the study, and second, patients were prescribed topical corticosteroids rather than antihistamines to treat pruritus. The use of topical corticosteroids in these studies was therefore higher in the control group and accordingly there was a greater likelihood that treatment-related differences would reach statistical significance.
The similarities in the overall SCORAD score and the pruritus subscore in the PL extract and placebo groups indicates that PL extract may control pruritus more effectively than antihistamines on demand and that it also may reduce consumption of these drugs. The beneficial effect of PL extract was noted after the first month of treatment and became more noticeable in the last months of the study. The good safety profile of PL extract, which was not associated with a perceivable increase in risk under the study conditions, indicates that it can be prescribed as a long-term treatment. Finally, although we used a second-generation antihistamine (low penetration of blood-brain barrier), it should be noted that first-generation antihistamines are commonly used in routine practice; these cause more drowsiness and are associated with a higher risk of adverse effects in the pediatric population.7,8 Our results suggest that PL extract could reduce the risk of adverse effects associated with antihistamines by reducing the consumption of these drugs, although this would need to be confirmed in clinical trials specifically designed to test this hypothesis.
FundingSponsor: ASAC Pharma. Laboratorios Centrum S. A. Alicante.
This project was cofunded by the 2007-2013 Technological Fund within the European Regional Development Fund Operational Programme and the Spanish Centre for the Development of Industrial Technology (CDTI).
Conflict of InterestsDr. Ana Ramírez-Bosca is employed at ASAC Pharma.
Dr. Pedro Zapater has worked as a clinical researcher for ASAC Pharma, Novartis, MSD, Generfarma, and Atral.
Dr. Joaquín Díaz-Alperi is employed at ASAC Pharma.
Dr. José F. Horga has worked as a clinical researcher and given talks for ASAC Pharma, Novartis, MSD, Generfarma, Atral, Bioibérica, Abbott, Pfizer, and Lundbeck.
Dr. Isabel Betlloch, Dr. Francisco Albero, and Dr. Asunción Martínez report that they have no conflict of interests.
Francisco Albero: Dermatology, Hospital Virgen de los Lirios de Alcoy (Alicante, Spain). Milagros Azorín: Dermatology, Centro de Especialidades Babel de Alicante. Isabel Betlloch: Dermatology, Hospital General Universitario de Alicante. Pedro Devesa: Dermatology, Hospital General Universitario de Elche (Alicante, Spain). Joaquín Díaz-Alperi: ASAC PHARMA, Alicante. Pedro M. Genovi: Dermatology, Hospital Marina Baixa (Alicante, Spain). José F. Horga: Unidad de Farmacología Clínica, Hospital General Universitario de Alicante e Instituto de Bioingeniería de la Universidad Miguel Hernández (Elche, Alicante, Spain). Asunción Martínez: Dermatology, Centro Dermatológico Estético de Alicante. Concha Más: Dermatology, private practice (Alicante, Spain). Conrad Pujol: Dermatology, Hospital Universitario La Fe, Valencia. Ana Ramírez: Dermatology, Centro Dermatológico Estético de Alicante. Eugenio Salgado: Dermatology, private practice (Alicante, Spain). Pedro Zapater: Clinical Pharmacology Unit, Hospital General Universitario de Alicante, Instituto de Bioingeniería, Universidad Miguel Hernández (Elche, Alicante, Spain).
At the end of the article is attached an Annex 1 with the members of the working group.
Please cite this article as: Ramírez-Bosca A, Zapater P, Betlloch I, Albero F, Martínez A, Díaz-Alperi J, Horga JF, y Grupo de Anapsos en Dermatitis Atópica y centros de realización del estudio. Actas Dermosifiliogr.2012;103:599–607.