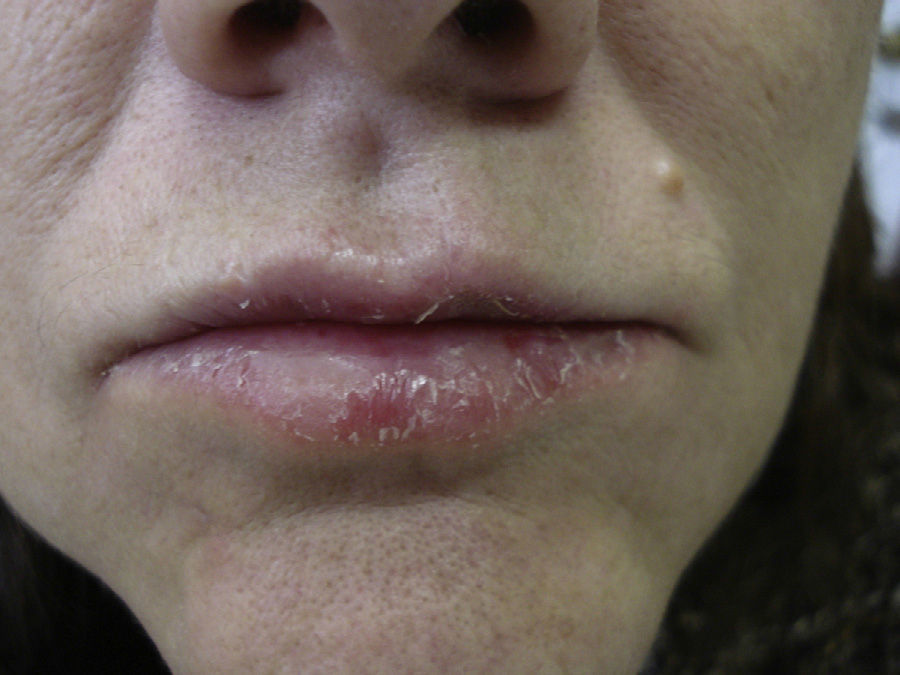
The patient was a 64-year-old woman referred to the skin allergy unit of our dermatology department with a 1-year history of chronic pruritic fissured cheilitis on the lower lip (Fig. 1). The physical examination also revealed dermatitis at the outer margin of the right lower eyelid that appeared in outbreaks, as well as cracked and dyshidrotic dermatitis on the tip of the right thumb that had been present for as long as the cheilitis.
Patch testing was performed with the standard series of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC) and a cosmetics series. The results were positive for cobalt chloride (+++) with no present relevance.
The patient had been taking Largactil drops (chlorpromazine) 5mg/24h to treat irritable bowel syndrome for 1 year.
Photopatch testing was performed with chlorpromazine 0.1% in petrolatum (irradiation, 5J/cm2). The result for the patch was negative (–), and that of the photopatch was positive (++). Phototesting was not performed. Given the suspicion of contact photoallergy to chlorpromazine, the drug was switched to levopromazine after patch testing with levopromazine at 1% and 0.1% in petrolatum (patch and photopatch negative).
The patient was free of lesions at a follow-up visit a few weeks later. The condition has been controlled for more than 4 years, with no new outbreaks.
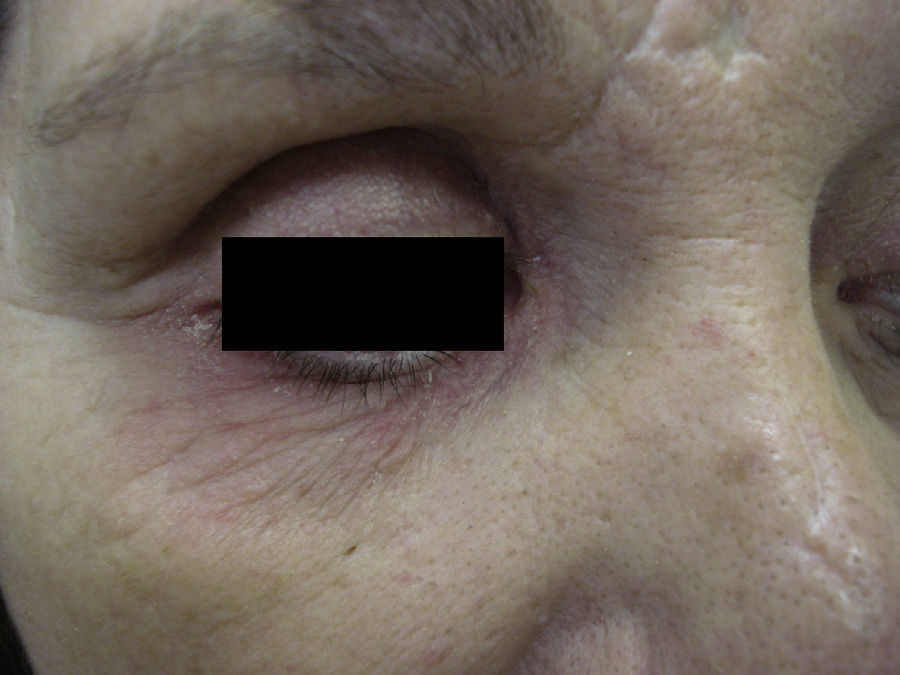
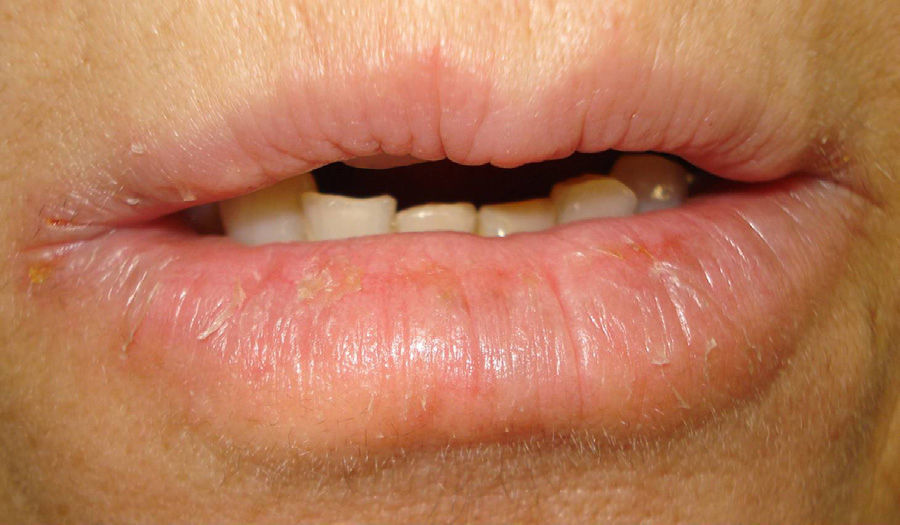
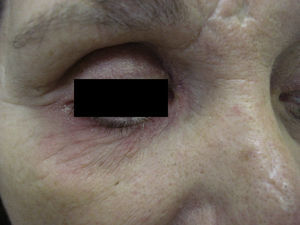
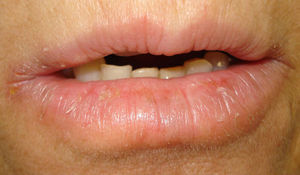
Case 2A 52-year-old woman was referred for possible contact dermatitis on the right lower eyelid that had begun 2 years previously (Fig. 2). She also reported pruritic chronic cheilitis that was sometimes cracked and painful and dated from the same period (Fig. 3). She wore a polymethyl methacrylate ocular prosthesis as a result of enucleation of the right eye after a traffic accident. She was taking eyedrops (Oftalmosa Cusí hydrocortisone ophthalmic ointment, Terramycin ophthalmic ointment 1%, Oftalmowell eyedrops, and Hidrathea eyedrops), as well as several drugs to treat brain injury sequelae resulting from the accident.
Phototesting was performed, as was patch testing with the standard GEIDAC series, a cosmetic series, an acrylate series, and the patient's own products, including all the ophthalmic ointments and eyedrops. The results were positive for paraphenyldiamine and mercury, with no present relevance.
Further questioning revealed that one of the drugs she was taking was Largactil drops 3mg/d.
Given our experience with patient 1, we performed photopatch testing with 0.1% chlorpromazine in petrolatum+UV-A (irradiation, 5J/cm2). The result of the patch was negative (–), and that of the photopatch was positive (++). In the phototest, which was performed while the patient was taking chlorpromazine (3mg/d), the minimal erythema dose for UV-B (22.7mJ/cm2) and the response to UV-A were considered normal for the patient's skin type (II) and according to local phototesting values.1
We also observed dermatitis on the tip of the index finger of the right hand, which was the one used to open the Largactil container, and a punctiform eczematous lesion on the palm, where she sometimes placed the drug before taking it. The patient also reported symptoms of photosensitivity at the onset of her condition (when she was taking chlorpromazine [18mg/d]), which had not appeared since the dose was reduced to 3mg/d. Chlorpromazine was switched to levopromazine after photopatch testing with levopromazine at 1% and 0.1% in petrolatum (patch and photopatch negative).
The patient was free of lesions at the follow-up visit 15 days later and has remained asymptomatic for the last 4 years.
DiscussionChlorpromazine is a classic antipsychotic drug of the aliphatic phenothiazine class. It can cause allergic contact dermatitis, especially phototoxic and photoallergic reactions. In fact, it is the archetypal photosensitizing drug. Chlorpromazine has been reported to affect patients who take it and persons who handle it, for example, those caring for people treated with chlorpromazine or persons exposed to the drug in the workplace (eg, nurses, pharmacists, and vets).2–4 In the review of photopatch testing performed by the Spanish Photobiology Group, chlorpromazine 0.1% was positive only twice, and its relevance was unknown.5
We present 2 cases of photoallergic contact dermatitis in women taking chlorpromazine. There are no previous reports in the literature of photoallergic contact dermatitis manifesting as chronic fissured cheilitis and caused by chlorpromazine. Both patients had dermatitis on the tips of the fingers used to open the container. This manifestation has been described elsewhere.3 In addition, both patients had occasional or continuous outbreaks of eyelid eczema on the side of the dominant hand.
Both patients only presented symptoms of contact photosensitivity to the drug despite systemic exposure.6 The workup should have been completed with phototesting in patient 1 while she was taking the drug in order to ensure the absence of photosensitivity.
Lastly, we also showed that there was no cross-photosensitization between the aliphatic phenothiazines chlorpromazine and levopromazine; therefore, this drug could be a good option in patients with symptoms of photosensitivity caused by chlorpromazine.
We are grateful to Nurse Imma Sierra Talavantes for her incalculable assistance in the diagnostic tests.
Please cite this article as: Esteve-Martínez A, Ninet Zaragoza V, de la Cuadra Oyanguren J, Oliver-Martínez V. Queilitis fotoalérgica de contacto por clorpromazina: descripción de 2 casos. Actas Dermosifiliogr. 2015;106:518–520.