Adjuvants are commonly incorporated into licensed vaccines to enhance the host immune response to their antigens. Aluminum salts, first introduced as vaccine adjuvants in 1926, remain widely used. Although generally safe, aluminum-containing adjuvants have been rarely linked to complex local adverse reactions, presenting with diverse clinical features and histologic patterns. These events are reported more often after subcutaneous or intradermal administration than after deep intramuscular injection.1 We describe a rare case of panniculitis in a 5 year-old child that occurred 5 days after administering the diphtheria–tetanus–pertussis (DTP) vaccine with an atypical extension.
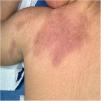
Case reportA 5-year-old patient, with no past medical history, and up-to-date immunizations per the national schedule presented to our department with a newly developed erythematous subcutaneous nodule. The lesion was located over the deltoid region at the site of the 2nd DTP booster injection, extending posteriorly along the ipsilateral shoulder to the upper back. Skin examination revealed the presence of an erythematous, indurated, and infiltrated plaque on the lateral area of the left arm with extension to the back (Fig. 1). Histologic examination revealed a non-specific inflammatory infiltrate within the hypodermis, involving both septa and lobules, consistent with a mixed panniculitis. The patient was put on dexamethasone 2mg twice a week. The course of the disease was favorable with confirmed improvement 1 month into therapy and complete resolution of the lesion 2 months into therapy with minimal residual atrophy and pigmentary changes.
DiscussionDTP vaccine is one of the vaccines containing aluminum salts.2 The latter are known to work through depot formation at the injection site leading to antigen persistence, and improved attraction of and uptake by APCs (antigen-presenting cells) which increase the effectiveness of vaccines by potentiating the immune response.2 Side effects seem to be due to the overactivation of immune responses leading to the processing and release of pro-inflammatory type I cytokines, such as IL-1, TNF-alpha, IFN- beta, IFN-gamma, IL-6, and IL-8.2
Several local reactions have been reported following DTP vaccine such as local pain, pruritus, erythema, cysts, sterile abscess and subcutaneous nodules.1,2 Although aluminum allergy has been proposed by some authors, the exact cause of these cutaneous reactions to DPT and other aluminum-containing vaccines remains unclear. Intramuscular injections are less likely to cause this type of reaction vs subcutaneous injections.1 The interval between vaccination and the onset of cutaneous signs ranges from several days to a few weeks after immunization.3 In some cases, however, a much longer latency has been reported, extending from months to even years.1,4 In a series of 14 cases on the different histiological aspects of post-vaccine nodules containing aluminum salts, several variants were described namely panniculitis, pseudolymphoma, necrotizing granuloma and lupus pronfondus-like.5 The typical histological appearance found in all cases was the presence of histiocytes with violaceous granular cytoplasm, as in our patient.
The particularity of our case was the spread of the lesions toward the back, at a distance from the injection site of the vaccine. We can probably explain this loco-regional extension of the inflammatory process by the spread of proinflammatory cytokines through the lymphatic or blood circulation.2
Since the benefits of these vaccines are far higher than the risks involved, aluminum-containing vaccines continue to be used. Therefore, to minimize the risk of these effects, deeper intramuscular injections are more recommended.
Author contributionsAll authors have read and confirm final version of the article.
Statement of ethicsThe patient's consent was obtained for the pictures and eventual publication.
Funding sourcesThe authors did not receive any funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are indebted to the patient for giving us the consent for publication.