Ruxolitinib is an oral inhibitor of Janus Kinase (JAK) 1 and 2 which has been approved for the treatment of polycythemia vera (PV), myelofibrosis (MF), and graft-versus-host disease (GVHD). It is also used off-label for chronic neutrophilic leukemia (CNL).1,2 Since its commercialization, safety reports have revealed an increased risk of non-melanoma skin cancer (NMSC), particularly cutaneous squamous cell carcinoma (cSCC).3,4 Additionally, several published cases point to greater aggressiveness in ruxolitinib-associated cSCC.1,5
Given the growing dermatological indications for ruxolitinib, we conducted a retrospective bicentric study including patients who developed any cSCC while on ruxolitinib. The study was approved by the Drug Research Ethics Committee (CEIM). Clinical, pathological, and evolutionary characteristics were collected from each patient. Descriptive analysis of qualitative variables was expressed as absolute frequencies and quantitative variables as median (interquartile range).
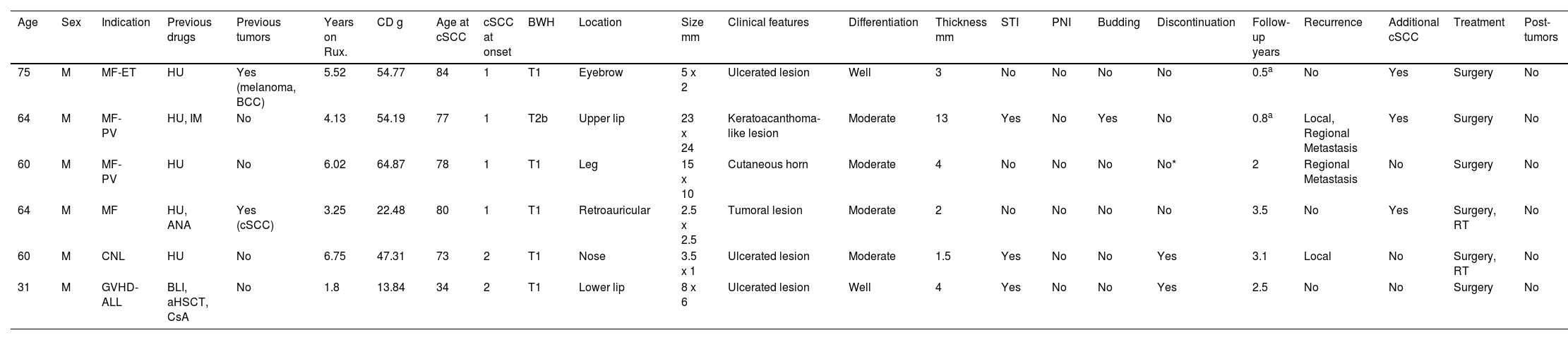
A total of 6 patients were identified who had developed, at least, 1 cSCC while on ruxolitinib (Table 1). All patients were men, with 4 receiving the drug for MF, 1 for GVHD, and 1 for CNL. The median age at diagnosis of the underlying disease was 62 years (range, 52–66). Five had previously been treated with hydroxyurea. Two cases had a past medical history of skin cancer. Patients developed cSCC after a median of 4.8 years (range, 2.9–6.2) on ruxolitinib, with a cumulative median dose of 50.8g (range, 20.2–57.9g) and a median age of 77.5 years (range, 67–81).
Clinical-pathological characteristics of the cutaneous squamous cell carcinomas in the series.
| Age | Sex | Indication | Previous drugs | Previous tumors | Years on Rux. | CD g | Age at cSCC | cSCC at onset | BWH | Location | Size mm | Clinical features | Differentiation | Thickness mm | STI | PNI | Budding | Discontinuation | Follow-up years | Recurrence | Additional cSCC | Treatment | Post-tumors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 75 | M | MF-ET | HU | Yes (melanoma, BCC) | 5.52 | 54.77 | 84 | 1 | T1 | Eyebrow | 5 x 2 | Ulcerated lesion | Well | 3 | No | No | No | No | 0.5a | No | Yes | Surgery | No |
| 64 | M | MF-PV | HU, IM | No | 4.13 | 54.19 | 77 | 1 | T2b | Upper lip | 23 x 24 | Keratoacanthoma-like lesion | Moderate | 13 | Yes | No | Yes | No | 0.8a | Local, Regional Metastasis | Yes | Surgery | No |
| 60 | M | MF-PV | HU | No | 6.02 | 64.87 | 78 | 1 | T1 | Leg | 15 x 10 | Cutaneous horn | Moderate | 4 | No | No | No | No* | 2 | Regional Metastasis | No | Surgery | No |
| 64 | M | MF | HU, ANA | Yes (cSCC) | 3.25 | 22.48 | 80 | 1 | T1 | Retroauricular | 2.5 x 2.5 | Tumoral lesion | Moderate | 2 | No | No | No | No | 3.5 | No | Yes | Surgery, RT | No |
| 60 | M | CNL | HU | No | 6.75 | 47.31 | 73 | 2 | T1 | Nose | 3.5 x 1 | Ulcerated lesion | Moderate | 1.5 | Yes | No | No | Yes | 3.1 | Local | No | Surgery, RT | No |
| 31 | M | GVHD-ALL | BLI, aHSCT, CsA | No | 1.8 | 13.84 | 34 | 2 | T1 | Lower lip | 8 x 6 | Ulcerated lesion | Well | 4 | Yes | No | No | Yes | 2.5 | No | No | Surgery | No |
ANA: anagrelide; CD: cumulative dose; MF: myelofibrosis; ET: essential thrombocythemia; HU: hydroxyurea; BCC: basal cell carcinoma; IM: imatinib; cSCC: cutaneous squamous cell carcinoma; BWH: Brigham and Women's Hospital classification system for cSCC; STI: subcutaneous tissue infiltration; PNI: perineural invasion; BLI: blinatumomab; aHSCT: allogeneic hematopoietic stem cell transplant; CSA: cyclosporine a; RT: radiotherapy; GVHD: graft-versus-host disease; ALL: acute lymphocytic leukemia; CNL: chronic neutrophilic leukemia; PV: polycythemia vera; Rux: ruxolitinib; m: male.
At diagnosis, 2 patients exhibited 2 cSCCs, most usually located in the facial area (lip). According to the Brigham and Women's Hospital classification system for cutaneous squamous cell carcinomas, 5 cases were T1 and 1 was T2b.6 Clinical presentation was heterogeneous, with ulcerated lesions being the most common, and median size, 6.5mm × 4.2mm. In 4 cases, differentiation grade was moderate. The median tumor thickness was 3.5mm (range, 1.8mm up to 6.2mm). In 3 cases, there was infiltration of subcutaneous tissue; no cases showed perineural infiltration, and 1 case exhibited tumor budding. The drug was discontinued in 2 cases, by mutual agreement with the patient. After a median follow-up of 2.25 years (range, 0.72–3.2), 1 local and 2 metastatic recurrences were reported, one to the skin and the other to the lung. Three patients developed additional cSCCs. All patients were treated surgically, and 2 were also put on radiotherapy. Two patients died, one from pulmonary metastases of cSCC and the other from their underlying disease.
This case series systematically presents the clinical and pathological characteristics of cSCCs in patients on ruxolitinib, including the first reported patients in the literature with GVHD and CNL.
High-risk factors for the development of regional or distant metastases in cSCC include location on the lip or ear, the presence of multiple cSCCs, recurrence, immunosuppression, size > 2cm, perineural invasion, poor differentiation, and, recently, tumor budding.7 Although the clinical-pathological features of the primary tumors in this series were not classified as high-risk according to current classification systems, it is notable that 2 of the 6 cases developed distant metastases during follow-up, suggesting increased aggressiveness.
The first studies to highlight an elevated risk of NMSC associated with ruxolitinib were randomized clinical trials that demonstrated its efficacy in MF and PV.3,4 On the other hand, ruxolitinib has not shown an increase in NMSC in GVHD or CNL.8,9 Subsequently, 6 cases and a 5-case series of of cSCC during drug exposure were published, where the authors warned of its aggressive nature (Supplementary data, Table 1).
Several factors could be responsible for the higher cSCC rate reported in these patients, notably HU. To determine the real influence of the drug, a 10-year retrospective cohort was conducted, including 188 patients with PV/MF exposed to ruxolitinib and 376 non-exposed. After adjusting for hydroxyurea, hazard ratio (HR) associated with ruxolitinib was 2.69 (95%CI, 1.03–7.02) for NMSC and 3.24 (95%CI, 1.45–7.22) for cSCC.10
The underlying reason for this risk is unclear. Ruxolitinib decreases the number and activity of NK cells, dendritic cell function and migration, T-cell numbers, and inflammatory interleukins. Therefore, immune-oncological surveillance may be impaired.1,2 On the other hand, the decision to discontinue the drug is controversial. Some authors choose to stop it, while others maintain it in cases where the underlying disease is uncontrolled and associated with metastatic cSCC, combining it with anti-PD1.1,11
Since JAK inhibitors are emerging as first-line therapies for some of the most common dermatological conditions, more evidence is needed to establish the potential increased risk of developing aggressive cSCCs in these patients. The decision to discontinue the drug should be individualized.
Conflicts of interestNone declared.
We wish to thank Dr. Virginia Velasco Tirado, Dr. Mónica Pozuelo Ruiz, Dr. Alberto Romo Melgar, Dr. Carlos Abril Pérez, Dr. Concepción Román Curto, Dr. Miguel Navarro Mira, Dr. Francisco Domínguez de Luis, and Dr. Begoña Escutia Muñoz for their contributions in providing cases and follow-up of these patients.