Oral lichen planus (OLP) is a relatively common inflammatory disease with a wide range of clinical forms. Its pathogenesis has not been fully elucidated although it is known to be mediated by lymphocytes with the participation of cytokines and other inflammatory cells, including type I and type II dermal dendrocytes (DD) (factor XIIIa+ DD and CD34+ DD, respectively).
ObjectivesTo describe the presence and tissue distribution of these cells, through immunohistochemistry, in 23 specimens from patients with clinical and histopathological criteria of OLP.
ResultsFactor XIIIa+ DD were mainly located in the superficial dermis (p<0.0001) as opposed to the deep submucosa. These cells were abundant throughout the dermal-epidermal junction and closely related to lymphocyte infiltration. Moreover, factor XIIIa+ DD were also found in the epithelium and deep dermis. CD34+ DD were distributed mostly to the deep dermis directly below the lymphocyte infiltrate with few cells in the subepithelial region.
ConclusionsDD were present in OLP, with distinct tissue distributions. Factor XIIIa+ DD were predominant in the superficial dermis while CD34+ DD could be found mostly in the deep dermis. These findings suggest that DD, and those positive for factor XIIIa+ in particular in view of their ability to express intercellular adhesion molecule-1 (ICAM-1) and tumor necrosis factor α (TNF-α), may play an important role in pathogenesis of OLP.
El liquen plano oral (LPO) es una enfermedad inflamatoria relativamente frecuente que se presenta con un amplio abanico de formas clínicas. Todavía no se ha determinado completamente su patogenia, aunque se sabe que los linfocitos actúan de mediadores con la participación de citoquinas y otras células inflamatorias, entre ellas los dendrocitos dérmicos (DD) tipo I y tipo II (DD positivos para el factor XIIIA y CD34, respectivamente).
ObjetivosDescribir la presencia y distribución de estas células en el tejido, mediante técnicas inmunohistoquímicas, en 23 muestras procedentes de pacientes que reunían los criterios clínicos e histopatológicos de LPO.
ResultadosLos DD factor XIII+ estaban localizados principalmente en la dermis superficial (p < 0,0001) y no en la submucosa profunda. Dichas células se encontraban en abundancia en toda la unión dermoepidérmica y se relacionaban estrechamente con la infiltración linfocitaria. Los DD factor XIIIa+ se encontraban además en el epitelio y la dermis profunda. En cambio, los DD CD34+ se distribuyeron principalmente en la dermis profunda, directamente por debajo del infiltrado linfocitario, con pocas células en la zona subepitelial.
ConclusionesLos DD estaban presentes en el LPO, con diferentes distribuciones en los tejidos. Así, los DD factor XIIIa+ predominaban en la dermis superficial, mientras que los DD CD34+ se encontraban principalmenteen la dermis profunda. Esto apunta a que los DD, y sobre todo los DD factor XIIIa+ debido a su capacidad para expresar moléculas de adhesión intercelulares-1 (ICAM-1) y el factor de necrosis tumoral alfa (TNF-α), pueden desempeñar una función destacada en la patogénesis del LPO.
Oral lichen planus (OLP) is a chronic and relatively common inflammatory disease. Unlike cutaneous lichen planus, which is generally self-limited and accompanied only by itching, OLP has a chronic course with rare spontaneous remission, and is often accompanied by discomfort and pain, with a potential for malignant transformation1,2.
Its etiology remains obscure. However, recent studies suggest that immunological mechanisms are fundamental for the onset and perpetuation of the clinical picture.
Dermal dendrocytes (DD) are cells derived from the bone marrow. They are distinct from Langerhans cells that present characteristics similar to mononuclear phagocytes (monocyte/macrophage). Basically two types of DD were identified by immunohistochemical study: factor XIIIa+, also called DD type I, and CD34+, or DD type II3,4.
Factor XIIIa is a plasma trans-glutaminase. It is important for the coagulation sequence, production of collagen by fibroblasts and connections between fibrin, fibronectin and collagen. These FXIIIa+ cells were initially observed around the portal space5. At first, because of their spindle cell morphology, these cells were considered to be fibroblasts. In 1986, Headington described dendritic cells in the normal dermis with histoenzymatic, immunohistochemical, and ultra-structural characteristics that are different from fibroblasts and Langerhans cells. He named those cells dermal DD6. Later Cerio et al detected factor XIIIa that correlated to the DD described by Headington and, based on this, FXIIIa was considered the immunohistochemical marker for DD3.
Other authors observed that most of these cells, besides present in the papillar and upper reticular dermis, presented a narrow relationship with the blood vessels and had a morphologic aspect similar to the phagocytic mononuclear tissue (macrophage/monocyte)7. Those cells would be derived from bone marrow (HLE-1+) and co-expressed markers for cells presenting antigens, macrophages or monocytes (human leukocyte antigen [HLA]-DR+, lymphocyte function antigen [LFA]-1+, HLA-DQ+, OKM5, Mo1+, Mono-1+, Leu M3+)8. For some authors, DD would be immature precursors of Langerhans cells9, however as the macrophages, they do not show Birbeck granules at electron microscopy study (ultra-structural aspect), speaking against that hypothesis10. The characteristics of factor XIIIa+ DD are described in table 1.
In normal skin and oral mucosa FXIIIa+ DD are located in the upper dermis and corion in association with collagen, and especially around blood vessels11. In inflamed skin, they are also found in the dermal and epidermal inflammatory infíltrate, and in the epidermis in association with lymphocytes8,10.
Many authors are studying the immunological function of dermal DD. Cerio et al suggest that dermal DD comprise a multi-potential population with capacity to exercise the function of macrophages, cells presenting antigens, and may differentiate into Langerhans cells when they migrate to the epidermis. These authors, in 1989, observed that, in the skin with inflammation, DD expressed intercellular adhesion molecule-1 (ICAM-1) after stimulation by interferon-γ (IFN-γ)8. Its phagocytic capacity was demonstrated by Headington6, and proven by Cerio et al who also observed proliferation of those cells in inflammatory processes11. Despite not proven, DD can be capable of phagocytosis of antigen-antibody complexes12 and in vitro they are as powerful as the Langerhans cells in the capacity of antigen presentation13. There is also a suggestion that DD would stimulate the migration of T lymphocytes, through the production of tumor necrosis factor α (TNFα)14. DD seem to have a close relation to mastocytes, both in relation to their location near those cells in the dermis, as to its possible activation and proliferation after degranulation of the mastocytes and release of TNFα15,16. DD also have an in vitro capacity to alter their shape to rounded cells similar to monocytes; and again mastocytes seem to be involved in this process.17 The main immunological functions of DD are listed in table 2.
Possible immunological functions of DD
| Antigen presentation |
| Differentiation to Langerhans cells? |
| Increase of the ICAM-1 expression |
| Stimulus for LT migration |
| Stimulus for TNFα expression? |
| Phagocytic capacity? |
DD: dendrocytes; ICAM-1: intercellular adhesion molecule-1;
LT: T-lymphocytes; TNFα: tumor necrosis factor α.
Regezi et al, in 19924, observed another dendritic cell, similar to factor XIIIa+ DD in the normal deep corion. This cell presented antigen for CD34, a glycoprotein found in the hematopoietic progenitor cells. In the dermis, CD34 is expressed by endothelial cells, cells of fusiform aspect around anexial structures and dendritic cells located in depth4,18. The function of these cells still remains obscure and there are no clear indications that these cells contribute to the inflammatory process. For some authors, the CD34+ cells could represent a reservoir of multipotential stromal cells with capacity for tissue migration during inflammation and repair19.
Other antigenic markers for DD, besides factor XIIIa+, as HLA-DR, HLA-DQ Leu M3 (CD14), CD36 (OKM5), Mono1, Mo1 are also described, however they are less specific8.
Recently, Monteiro et al detected, using the immunochemistry, the GPIb3a receptor of the von Willebrand factor (vWF), that according to the authors would be a more specific and sensitive marker for the dendrocytic dermal factor XIIIa+17. Those same authors demonstrated greater expression of the GPIbα receptor in the DD after mastocyte degranulation possibly by a mechanism independent of TNFα.
Recent studies have been attempted to relate those cells with inflammatory processes of immunological origin, among them OLP, and thus elucidate some obscure points in the pathophysiology of that disease.
Due to the participation of DD in the physiopathology of inflammatory processes mediated by T lymphocytes (LT) we believe that DD have an important role in OLP.
Material and methodsPatientsA retrospective study was accomplished with 23 biopsies from patients with a clinical and histopathological diagnosis of OLP.
Selection of materialThe biopsies came from the Sector of Pathology of the School of Medicine and HUCFF/UFRJ, of the Federal University of Rio de Janeiro.
The presence of at least 3 of the 5 following histopathological alterations were used as criteria for the histopathological diagnosis of OLP: a) dyskeratosis (hypergranulosis and/or parakeratosis); b) necrosis of keratinocyte (Civatte bodies); c) lichenoid infíltrate; d) vacuolation of the basal layer, and e) pigmentary incontinence.
The histologic sections that contained little corion (conjunctive stroma underlying the epithelium of the mucosa) were discarded, because the immunohistochemical technique for the DD would be impaired.
Primary antibodiesThe primary antibodies were, for factor XIIIa, a polyclonal antibody produced in rabbit (Calbiochem-Novabiochem Co; La Jolla, CA) in a dilution of 1:200, and for CD34, a monoclonal antibody produced in mouse (HPCA-1, Becton-Dickinson, San Jose, CA) in a dilution of 1:50.
Immunohistochemical methodImmunohistochemical staining was carried out as per the manufacturer's instructions.
Immunohistochemical analysisCases in which at least four histological fields with a 40x magnification could be analyzed were considered. For purposes of an approximate quantification of the infiltrate we adopted the following classifications:
- 1.
Scattered - less than 20 cells in the 4 fields.
- 2.
Concentrated - 21 or more cells in the 4 fields.
Statistical analysis was accomplished by the Fisher exact test for association between qualitative variables and the McNemar test to verify if significant variation in the factor XIIIa (or CD34) count from one position to the other exists. The criterion for determination of adopted significance was a 5 % level. The statistical analysis was processed using SAS™ System statistical software.
ResultsFXIIIa+ and CD34+ DD were recognized by presence of brown and red coloration, respectively, in the cytoplasm of the cells with dendritic morphology. The semiquantification of the infiltrate is described in tables 3 and 4.
Numeric and percentage distribution of factor XIIIa+ DD in 19 analyzed samples
| FXIIIa+ DD | Superficial corion | Deep corion | ||
| Semiquantification of the infiltrate | Frequency | Percentage | Frequency | Percentage |
| Concentrated | 18 | 94.7 | 5 | 26.3 |
| Scattered | 1 | 5.3 % | 14 | 74.7 |
Frequency missing = 4. DD: dermal dendrocytes.
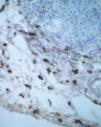
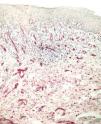
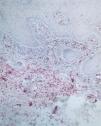
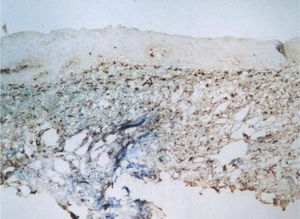
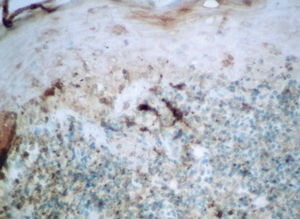
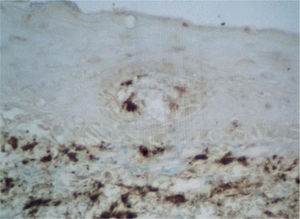
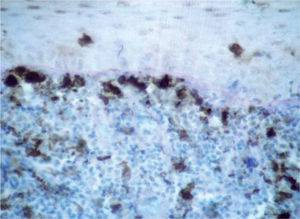
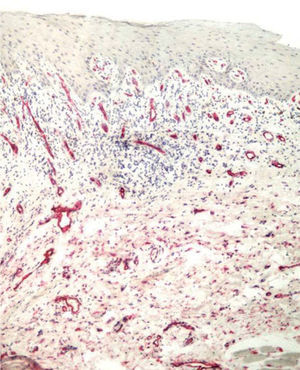
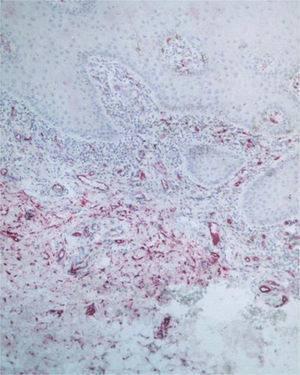
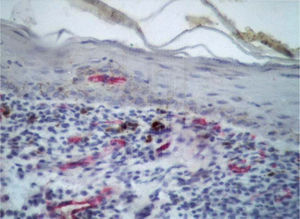
Factor XIIIa+ DD were concentrated in the superficial corion (figs. 1 and 2), mainly in a sub-epithelial position, in some cases paving this area (fig. 3). Some cells were also observed in the lower corion (fig. 4) and in the epithelium (fig. 5). Of the 19 cases studied for factor XIIIa+ DD, in the superficial corion, 18 were considered as concentrated (94.7 %) and 1 case was described as scattered. It was observed that a significant variation (drop) exists (p < 0.0001) in factor XIIIa+ DD count in the deep corion (26.3 %) in relation to the superficial corion (94.7 %).
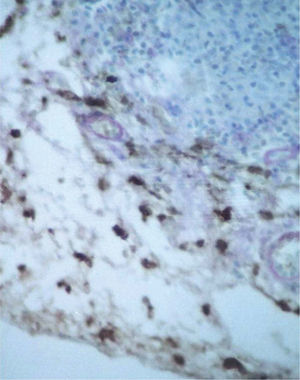
CD34+ DD were most often observed in the lower corion, right below the inflammatory infíltrate (figs. 6 and 7). Those cells were absent or were scattered in the superficial corion and around vassels (4.4 %) (fig. 8). It was observed that significant variation (increase) exists (p = 0,020) in CD34+ DD counting in the deep corion (22.7 %) in relation to the superficial (6.3 %).
For means of statistical analysis, the clinical forms were grouped into erosive and non-erosive forms, including reticular and plaque forms.
The statistical analysis of factor XIIIa+ and CD34+ DD's association with the clinical forms could not be accomplished regarding the upper corion since there was an absolute frequency of the number of increased cells (94.7 %).
In relation to the lower corion, as observed below (table 5), the group with erosive disease and that presents concentrated infíltrate in the lower corion, does not differ statistically from the group without erosive disease, related both to factor XIIIa as to CD34 DD, with p = 0.39 and 0.41 respectively.
DiscussionOLP is a relatively common disease, with different clinical patterns. Although the physiopathology is not entirely known, recent investigations suggest cell damage by lymphocytes, with participation of cytokines and adhesion molecules.
For many years, researchers have been questioning the importance of dermal dendritic cells in the natural immune response and, consequently, in the pathophysiology of certain diseases. The importance of the Langerhans cells is well known in the presentation of antigens and their crucial action in the activation of lymphocytes in the process of sensitization of allergic contact dermatitis20. However, little is known about the function of DD in the cutaneous immune process21. Identification of those cells in the inflamed skin8, points to their possible participation in the immunological processes.
We know that factor XIIIa+ DD are cells present in the corion and normal dermis and are increased in some diseases where a disturbance of the immune system and tissue repair processes occur10,11,16,22.
Regezi et al, in 1994, observed a significant statistical increase in numbers and size of factor XIIIa+ DD in the submucosa of 16 patients with OLP. Like us, they found most factor XIIIa+ DD in the upper corion23. Those authors used the adjacent non-compromised tissue as control for the histopathological aspects observed in OLP, and found an average of 10 cells in the upper and 8 in the lower corion, both characterized as scattered in the present investigation, which showed that the biopsies did not present lesion-free areas, with the totality of the samples compromised by the inflammatory infíltrate or by other characteristics that were adopted as criteria for histopathological inclusion. We prefer not to use the area adjacent to the lesion as control, even if histopathological alterations were not observed, since we cannot prove that that area, theoretically free from lesions, was also free from cytokine action, other mediators and adhesion molecules.
Recently, Deguchi et al described a similar pattern of factor XIIIa+ cells in the dermis of cutaneous lichen planus (CLP). Those researchers, however, as well as Regezi et al23, used the area adjacent to benign tumors for control, as seborrheic keratosis and melanocytic nevus21.
A significant increase of factor XIIIa+ DD (p < 0.0001) in the upper corion was observed. However, it was not possible to correlate this with the clinical form or histopathological aspects of OLP. In the present study, factor XIIIa+ DD were closely related to the inflammatory infíltrate and seemed to be part of it, different from what was reported by other authors, who described the great majority of those cells as located around the lymphocyte infíltrate without direct contact with it, while studying factor XIIIa+ DD in lesions of CLP and in the inflamed skin10,22. It was observed that regardless of the inflammatory infíltrate to be bundled or irregular, factor XIIIa+ DD were present in large numbers.
Like us, both Deguchi et al21 and Regezi et al4 described factor XIIIa+ DD in the epithelium in CLP and OLP. One hypothesis is that factor XIIIa+ DD are indirectly connected to apoptosis, possibly through either stimulation of TNF-α14, or exerting a function on antigen presentation and differentiation by Langerhans cells8.
If compared with what was considered normal by Regezi et al, in 1994, in the study discussed previously23, it could be stated that factor XIIIa+ DD were increased in numbers in the 19 analyzed biopsies, both in relation to the upper as lower corion. However, as already mentioned before, it is believed that the area adjacent to the lesion might be compromised and, in view of this, we chose to only describe factor XIIIa+ and CD34+ DD in the patients' submucosa with OLP.
In spite of not performing double staining for CD34 and factor XIIIa, the tissue distribution of DD confirms the hypothesis that they are different subsets of dendritic cells4,23.
In the normal submucosa, CD34+ DD are present in the lower corion. In the compromised mucosa, CD34+ DD distribution is similar to the normal mucosa4,18,24,25. The present study is the first to describe CD34+ DD distribution in the biopsies of OLP.
CD34+ DD are numerous immediately below the inflammatory infiltrate (p < 0.020). This suggests that these cells might be involved in the maintenance or stimulation of the inflammatory process. However, the possible immunological functions attributed to factor XIIIa+ DD, as antigen presentation13, T lymphocyte migration14 and expression of ICAM-1 and TNF-α15,16, were not described for CD34+ DD.
Conclusions- 1.
Factor XIIIa+ and CD34+ DD are present in OLP lesions and those cells have a distinct tissue distribution in OLP.
- 2.
Dendritic factor XIIIa+ cells prevailed in the upper portions of the corion, amongst the inflammatory infiltrate while CD34+ cells prevailed in deeper locations, below the inflammatory infíltrate. Factor XIIIa+ cells were also seen amongst keratinocytes and in the lower corion. CD34+ cells were observed, in smaller numbers, in the upper corion.
- 3.
Factor XIIIa+ and CD34+ DD distribution is not associated with the clinical forms nor with histopathological aspects of OLP.
Conflicts of interests
We declare that we have no conflict of interests.