Interleukin 31 (IL-31) is a neurocytokine that stimulates sensory neurons involved in pruritus. It contributes to skin barrier inflammation, dysfunction, and remodeling. As the immune and nervous systems are interrelated, IL-31 has a key role in the treatment of atopic dermatitis (AD) and prurigo nodularis (PN). Nemolizumab is a humanized monoclonal antibody that blocks the α subunit of the IL-31 receptor, modulates the neuroimmune response, and rapidly alleviates itching by directly blocking signaling. It reduces inflammation and lesion severity in AD and PN by restoring epithelial function and promoting skin barrier integrity. This review synthesizes the latest information on the functions of IL-31 and presents the current evidence, including clinical trial results, on the use of nemolizumab in the treatment of AD and PN.
La citoquina IL-31 es una neurocitoquina que estimula las neuronas sensoriales relacionadas con el picor, contribuye a la inflamación, la disfunción y remodelación de la barrera epidérmica. Al interrelacionar los sistemas inmunológico y nervioso constituye un factor clave en el tratamiento de dermatitis atópica (DA) y del prurigo nodular (PN). Nemolizumab es un anticuerpo monoclonal humanizado que bloquea la subunidad α del receptor de la IL-31 y modula la respuesta neuroinmunitaria, bloquea directamente la señalización del prurito y alivia rápidamente el prurito, controlando la inflamación y reduciendo la gravedad del eccema en DA y las lesiones pruriginosas del PN al restaurar la función epitelial y promover la integridad de la barrera cutánea.
Este artículo resume la nueva información relacionada con las funciones de la IL-31 y presenta la evidencia y resultados disponibles hasta el momento de los ensayos clínicos de nemolizumab en DA y PN.
Atopic dermatitis (AD) is a chronic inflammatory disease with a heterogeneous clinical presentation in which impaired skin barrier function, cutaneous dysbiosis, and an altered immune response lead to intensely pruritic eczematous lesions. These generate a cycle comprising scratching, itch, inflammation, and epithelial changes—the itch-scratch cycle—which hampers resolution of the disease.1–3 Prurigo nodularis (PN) is similar to AD in that it is characterized by onset of isolated, excoriated, and highly pruritic lesions, which are variable in number and diameter. Via an inflammatory process, the lesions are associated with sensitization of the neurons that process the itch and the development of an itch-scratch cycle that aggravates the disease.4,5
The cytokine interleukin (IL) 31 is a common link in these diseases. IL-31 is defined as a “pruritogenic cytokine” owing to its high tissue and serum levels in patients with allergic and pruritic diseases and to a potential link between the immunologic and neuronal systems that can induce pruritus. However, the effects of IL-31 are not limited to induction of pruritus, but rather comprise a broad spectrum that is involved in direct stimulation of the sensory neurons associated with itch. IL-31 acts as a proinflammatory and immunomodulatory cytokine that directly affects altered remodeling of epithelial tissue (itch-scratch cycle).6–12
Therefore, IL-31 has become a key clinical target for biologic drug therapy. Such is the case with nemolizumab, a human monoclonal antibody that blocks the α receptor of IL-31 (IL-31Rα), thus preventing the biologic activity of IL-31 and interrupting the itch-scratch cycle in associated diseases.13
IL-31 and the IL-31R ComplexIL-31 was first described by Dillon et al.14 in 2004. The authors observed that mice overexpressing IL-31 developed severe pruritus, alopecia, and skin lesions. IL-31 is a short-chain 4-helix cytokine belonging to the IL-6 cytokine family, which also includes leukemia inhibitory factor, oncostatin M (OSM), cardiotropin 1, IL-6, and IL-11. These cytokines participate in neuronal growth, bone metabolism, development of the heart, and immune regulation, as well as in T-cell differentiation.
IL-31 is secreted mainly by type 2 helper T (TH2) memory CD4+CLA+ cells6,14,15 and by other cell types such as macrophages, mastocytes, dendritic cells, eosinophils, and basophils.6
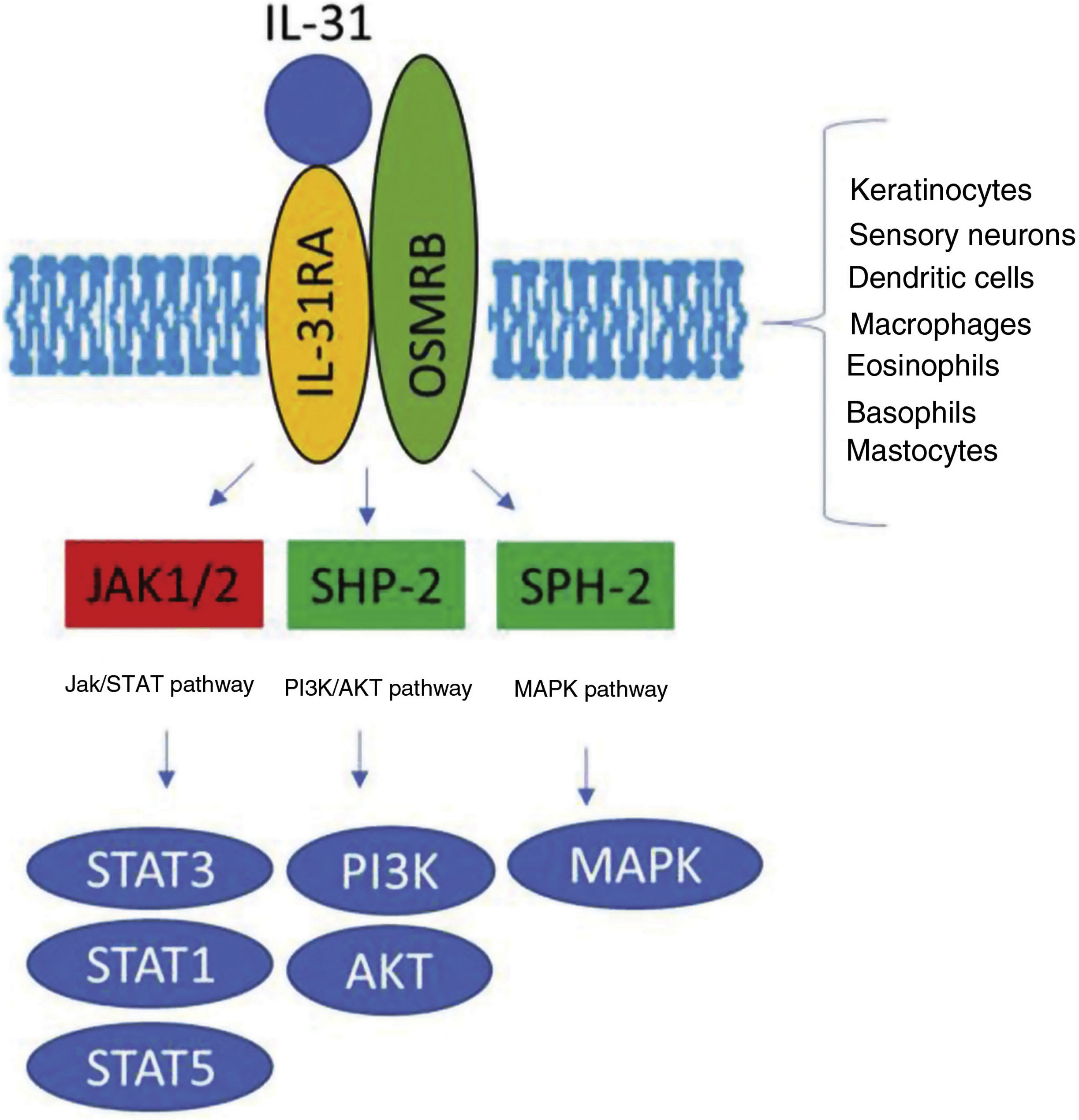
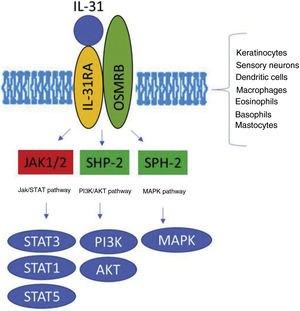
The IL-31 receptor (IL-31R) is a complex heterodimer formed by IL-31Rα and the oncostatin Mβ receptor (OSMR), whereas the remaining members of the IL-6 family share the glycoprotein 130 (gp130) chain. IL-31 binds to subunit α of IL-31R, but not to subunit OSMR in the IL-31R complex. However, after binding, OSMR converts IL-31R to a high-affinity receptor and increases binding of IL-31.7,11,16 Binding of IL-31 to this receptor results in the phosphorylation of a triple intracellular cascade, as follows: (a) Janus kinase 1 and 2 (JAK1/JAK2), together with their signal transducer and activator of transcription factors 1, 3, and 5 (STAT1, STAT3, and STAT5) (3 to a greater extent than 1 and 5); (b) the protein kinase B pathway (PI3K/AKT); and (c) the mitogen-activated protein kinase pathway7,9,13,16 (Fig. 1). IL-31R is expressed by a wide variety of epithelial, neuronal, and immune cells, such as keratinocytes, fibroblasts, dendritic cells, mastocytes, macrophages, basophils, and eosinophils, as well as by peripheral neurons and dorsal root ganglia (DRG). Therefore, its activation leads to direct and indirect effects on itch, inflammation, and skin barrier impairment.16–20
Transduction of the IL-31 signal via its receptor, IL-31R. IL-31 binds to subunit IL-31RA of the receptor and activates the intracellular cascade. IL indicates interleukin; OSMR, oncostatin M receptor; JAK, Janus kinase; SHP-2, SH2 domain containing protein tyrosine phosphatase; STAT, signal transducer and activator of transcription proteins; PI3K, phosphatidylinositol 3 kinase; MAPK, mitogen-activated protein kinase.
The sensation of pruritus in the skin is mediated by small nerve fibers, such as C fibers, which originate in the neurons of the DRG and run to the spinal cord, the hypothalamus, and, finally, the brain (the peripheral nerves do not express IL-31R). There are many pruritogens other than IL-31, for example, IL-13, IL-4, histamine, cathepsin S, and chloroquine.11,21,22 The DRG neurons express IL-4Rα for IL-4 and IL-13, which are also pruritogenic cytokines and increase the evoked action potential of histamine and IL-31, thus participating in the formation of itch.23
The itch induced by the histamine pathway (histamine/H1R) or chloroquine pathway (chloroquine/MrgprA3), for example, requires b-type natriuretic polypeptide (BNP), gastrin-releasing peptide (GRP), and their receptors to transmit the itch signal via the spinal cord. Itch induced by IL-31, on the other hand, requires neurokinin B instead of BNP to release GRP. It is perhaps for this reason that itch induced with IL-31 is not significantly attenuated with antihistamines, dexamethasone, or tacrolimus.11,24,25
In addition to mediating in the sensation of pruritus, IL-31 stimulates elongation and ramification of the small DRG neurons. This elongation is dependent on the activation of STAT3 and independent of transient receptor potential cation channel subfamily V member 1.11,26 This suggests that IL-31 can promote sensitivity to minimal stimuli such as maintained itch and contribute to the hypersensitivity that is characteristic of AD11,26 (Fig. 2).
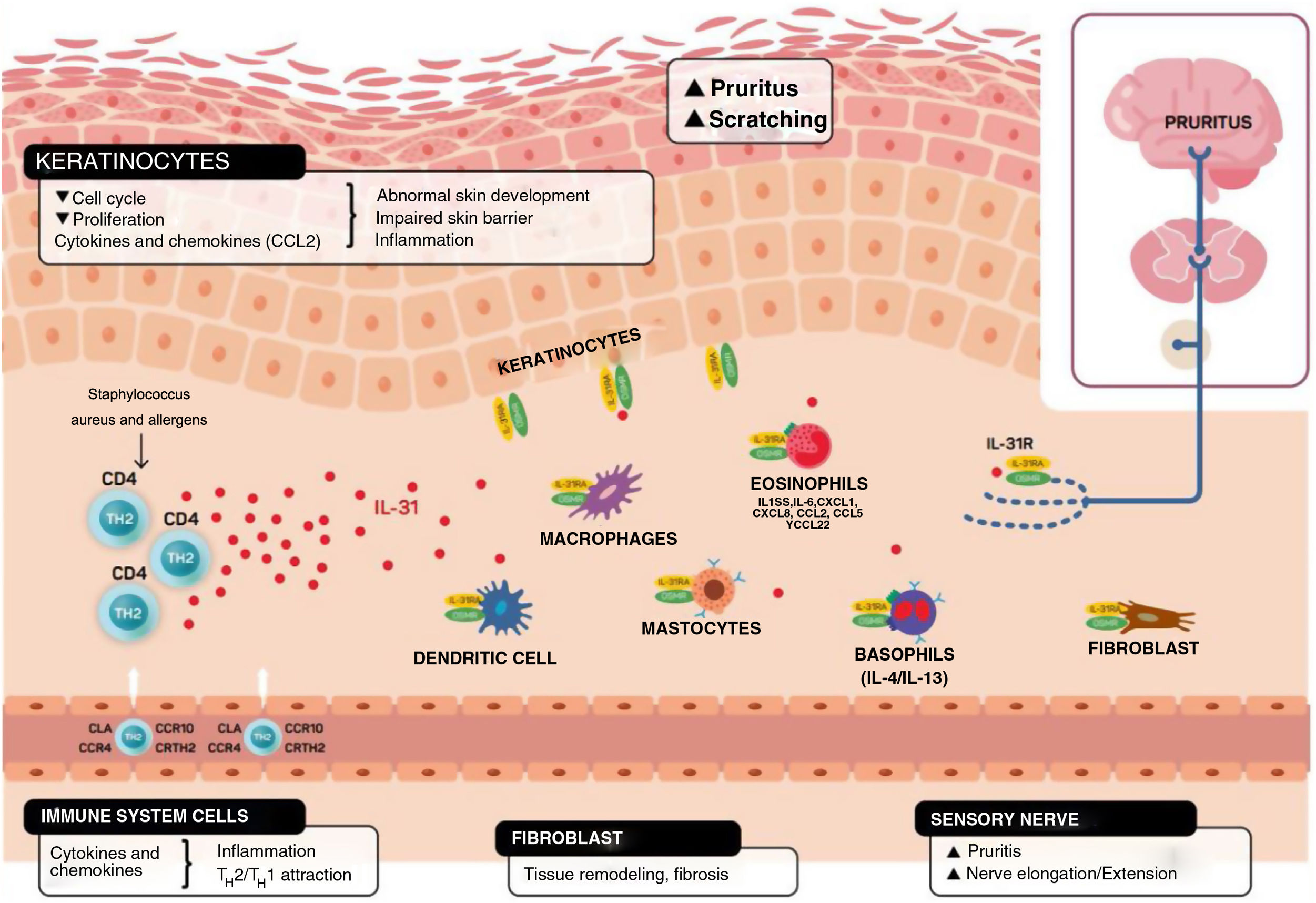
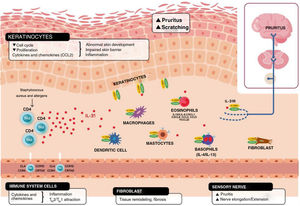
Schematic representation of the role of IL-31 in the skin. IL-31 is produced mainly by type 2 helper cells. Its receptor is a heterodimer of IL-31Rα and OSMRB expressed in multiple cells, including immune system cells, keratinocytes, and peripheral neurons. The itch-scratch cycle occurs during inflammation of the skin in atopic dermatitis. TH indicates helper T cell; IL, interleukin; CLA, cutaneous lymphocyte antigen; CCR, CC chemokine receptor; CR, chemoattractant receptor.
Keratinocytes in the skin express abundant IL-31Rα and OSMR.16 Here, IL-31 reduces the cell cycle and differentiation, as well as expression of barrier proteins such as filaggrin, involucrin, and cytokeratin 10. This alters skin barrier function and, in turn, increases expression of IL-1α, IL-20, and IL-24, which also alters skin barrier formation and enables penetration of allergens and irritants.
Furthermore, IL-31 boosts inflammation by inducing expression of chemokines and keratinocytes that attract TH2 memory lymphocytes, including CCL17/TARC and CCL22/MDC, as well as CCL19/MIP-3β, CCL23/MIP-3, and CCL4/MIP-1β (Fig. 2).
IL-31R is expressed on monocytes, macrophages, dendritic cells, eosinophils, mastocytes, and basophils. Activation of this receptor causes various effects. In basophils, stimulation by IL-31 leads to secretion of large amounts of IL-4 and IL-13 instead of histamine. This is relevant, since IL-4 leads to differentiation of T cells in TH2 (potent secretors of IL-31) and could act as an activation signal in type 2 skin inflammation. In eosinophils and dendritic cells, it induces secretion of a set of proinflammatory cytokines and chemokines (tumor necrosis alfa [TNF] α, IL-1β, IL-6, C-X-C motif chemokine ligand [CXCL] 1, CXCL8, C-C motif chemokine ligand [CCL] 2, CCL5, and CCL22), which is increased in the presence of keratinocytes but not fibroblasts.6,10,27 Via these molecules, IL-31 can attract neutrophils (CXCL1, CXCL8), dendritic cells (CCL2), TH1 (CCL5), and TH2 (CCL2) to the inflamed area and promote angiogenesis, inflammation (CXCL1, CXCL8), and/or tissue remodeling (CCL2, IL-6). Other cytokines (TNF-α, IL-1β, IL-6) can affect the functions of T cells, B cells, and dendritic cells, as well as activate neighboring epithelial cells. Several immune cells in turn produce IL-31. Eosinophils can also release IL-13 and augment this release in the presence of TH2 cytokines, mastocytes, basophils, macrophages, monocytes, and dendritic cells.6,28,29 In this way, inflammation by IL-31 can be amplified by several of these loops in immune cells.6 Tissue remodeling is a consequence of chronic inflammatory episodes associated with the TH2 response. AD, lichenified lesions have been subject to tissue remodeling. Expression of IL-31Rα is increased in fibroblasts in fibrotic tissue, as are IL-31 levels in the plasma of patients with fibrosis.30 IL-31 affects the expression of mechanisms of proliferation in genes and induction of collagen type 1 and dermal fibroblasts. Therefore, blocking IL-31 could prove beneficial in these situations.
Taken as a whole, IL-31 is a key regulator between the immune system, the nervous system, and the skin (Fig. 2).
Various molecules in different phases of research target IL-31. Paradoxically, lokivetmab, a canine monoclonal antibody aimed at blocking IL-31, is now being used in veterinary practice. It has been approved for treatment of pruritus associated with allergic dermatitis and with the clinical manifestations AD in dogs.31
As for research in humans, we now have data on 3 molecules: BMS-981164, vixarelimab, and nemolizumab, which is addressed in the present study. BMS-981164 is a discontinued antibody whose phase 1 clinical trial (NCT01614756) finished early, without publication of the results. Vixarelimab (KPL-716) is an anti-OSMR antibody that is currently being investigated and was shown to improve EASI and pruritus scores compared with placebo in a phase 1A/1B trial.32
Inhibition of IL-31 in Atopic Dermatitis and Prurigo Nodularis: NemolizumabItch is the most harmful symptom of AD and PN, leading patients to scratch persistently and enter the itch-scratch cycle. The direct consequences of this development are altered sleep patterns, quality of life, and daily living.2–5 Thus, in patients with moderate or severe AD and PN, the primary objective of therapy is to ensure well-being via control of itch, improvement in dermatitis (reducing inflammation and restoring the skin barrier function), and improvement in sleep quality and quality of life as final outcomes.
Nemolizumab is a human monoclonal antibody that, when administered subcutaneously, binds to the α subunit of the IL-31 receptor and blocks activation of the cell receptor. Published clinical evidence on this agent is now available,13,33–38 and the results reported are relevant (Table 1).
Summary of Clinical Trials with the IL-31R Inhibitor Nemolizumab: Phases 1, 2, and 3 in Atopic Dermatitis and Prurigo Nodularis.
| IndicationAuthor | Design | Inclusion criteria treatment/control, No. | Objective | Outcomes | Tolerability |
|---|---|---|---|---|---|
| Moderate to severe atopic dermatitisNemoto et al.13 | Phase 1 Dose escalation (0.003–3mg/kg). Phase 1B 0.3/1/3mg/kg single injection vs. PL | Poor control with previous topical treatment, BSA ≥5%, VAS pruritis ≥5, and IGA ≥327 NE/9 PL | SafetyTolerabilityEfficacy (VAS [pruritis], effective for sleep and use of hydrocortisone) | Good safety profile.Pruritus significantly diminished (−50% approx.) with NE, vs. PL (−20% approx.) at 4 wk;Improved sleep quality (+80% approx.) NE vs. PL (+50% approx.);Significant reduction in use of hydrocortisone with NE | Good safety profile with only 1 dose |
| Moderate to severe atopic dermatitisRuzicka et al.33 | Phase 2 0.1/0.5/2mg/kg every 4 wk and 2mg/kg every 8 wk vs. PL 12-wk follow-up | Poor control with previous topical treatment, EASI ≥10, VAS pruritis ≥5 and IGA ≥3216 NE/53 PL | Effective in VAS (pruritis), EASI, SCORAD, IGA, BSA, PVRS, SD-VAS, reduced use of TC | Pruritis significantly diminished −43.7% with 0.1mg/kg, −59.8% with 0.5mg/kg, and −63.1% with 2mg/kg every 4 wk and −20.9% with PL, at 12 wk.Significant improvement with NE for remaining parameters | Tolerability similar to that of PL. Exacerbation of AD and peripheral edema is more common with NE. |
| Moderate to severe atopic dermatitisKabashima et al.34 | Phase 2 0.1/0.5/2mg/kg every 4 wk and 2mg/kg every 8 wk vs. PL 52-wk follow-up (extension study of reference42) | Poor control with previous topical treatment, EASI ≥10, VAS pruritus ≥5 and IGA ≥3131 NE/37 PL | Effective in VAS (pruritus), EASI, SCORAD, IGA, BSA, PVRS, SD-VAS, reduced use of TC | Pruritus significantly diminished, −73% with 0.1mg/kg, −89.6% with 0.5mg/kg, and −74.7% with 2mg/kg every 4 wk and −79 with 2mg/kg every 8 wk at 64 wk. Diminished pruritus increased with time on treatment from 14 to 64 wk.The improvement was maintained or increased with NE in the remaining parameters at 64 wk. Improvement in EASI75 in more than 65% of patients with 0.1/0.5/2mg/kg every wk 4 and in EASI75 at 2mg/kg every 8 wk at 64 wk | Tolerability was maintained in the long term. The exacerbation of AD, nasopharyngitis, URTI, increased CK, peripheral edema, and injection site reaction were the most common findings with NE |
| Moderate to severe atopic dermatitisSilverberg et al.35 | Phase 2b 10/30/90mg every 4 wk vs. PL (with TC) 24-wk follow-up | Poor control with previous topical treatment, EASI ≥12, PPNRS ≥7, IGA=3–4, and BSA≥10%169 NE/37 PL | Efficacy demonstrated with EASIIGA, PPNRS BSA, SCORAD, sleep disturbance NRS, DLQI, EQ5D, HADS, reduced use of TC | Significant improvement in EASI. Dose of 30mg was most effective (−66.8% NE vs. 43.1% PL; P<.01 at 16 wk) (−68.8% NE vs. 52% PL; P=.016 at 24 wk) with significant differences from 8 wk onward (P≤.01). At 16 wk, EASI50 achieved in 59.6% vs. 36.8% (30mg NE vs. PL; P=.016) and EASI75 in 45.6% vs. 26.3% (30mg NE vs. PL; P=.014). At 24 wk, EASI50 in 66.7% vs. 43.9% (30mg NE vs. PL; P=.014) and EASI75 in 45.6% vs. 26.3% (30mg NE vs. PL; P=.014).Significant reduction in pruritus of −67.3% with NE 30mg and TC, and −35.8% with placebo and TC at wk 24; with differences from 1 wk onward 1 (P<.01).Improvement in IGA, PPNRS, Sleep Disturbance NRS, DLQI, EQ5D, and HADS. NE reduced the use of TC in 30–40% vs. PL. | Safety and tolerability profile similar to that of PL. Treatment-related AEs were nasopharyngitis and URTI. |
| Moderate to severe atopic dermatitisMihara et al.36 | Exploratory based on patients from phase 2 clinical trials 33,34 | Poor control with previous topical treatment, EASI ≥10, VAS pruritus ≥5 and IGA ≥3 | Improved productivity and activityExploratory analysis WPAI-AD | Significant improvement in workplace productivity and reduced impairment of activity at 64 wk. Improvements vs. PL observed as early as 4 wk and maintained from 12 wk to 64 wk. The improvement in WPAI-AD is correlated strongly with improvement in pruritus and sleep quality (PVRS, SD-VAS) | Not evaluated |
| Moderate to severe atopic dermatitisKabashima et al.37 | Phase 3 60mg vs. PL (with TC) 16-wk follow-up | IPR score ≥3, VAS pruritus ≥5, EASI ≥10143 NE/72 PL | Effectiveness by VAS pruritus, daily VAS pruritus (up to 4 wk), EASI, ISI, DLQI, NRS, POEM, WPAI-AD | Significantly diminished pruritus, −42.8% with NE 60mg and TC vs. −21.42% with PL and TC; P<.001 at 16 wk. Decreased EASI −49% vs. 33.2%; DLQI 40% vs. 21%, ISI 55% vs. 21%; NE and PL, respectively, at 16 wk. Significant change in VAS pruritus with NE from day 2 onward. | Tolerability similar to PL. Increased CK and injection site reaction were more frequent with NE |
| Moderate to severe prurigo nodularisStänder et al.38 | Phase 2 0.5mg/kg every 4 wk vs. PL 12 wk of follow-up | Phase 2 PPNRS ≥7; more than 20 nodules. 34 NE/36 PL | Efficacy PPNRS (mean and maximum) at 4 wk, PVRS, 7-Ítem Prurigo Score, IGA, DLQI | Significantly diminished pruritus, −53% with NE 0.5mg/kg and −20.2% with PL at 4 wk; P<.001. At 4 wk, 24% vs. 11% of patients (with 75% of lesions cured) and 29% of patients with no or almost no prurigo vs. 0% in NE vs. PL | Tolerability similar to PL. More digestive symptoms (abdominal pain and diarrhea) and musculoskeletal symptoms |
Abbreviations: BSA, body surface area affected; CK, creatine kinase; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; EQ5D, EuroQoL 5-Dimension; HADS, Hospital Anxiety and Depression Scale; IGA, Investigator Global Assessment; IPR, Inadequate Pruritic Response score; ISI, Insomnia Severity Index; NE, nemolizumab; PL, placebo; POEM, Patient-Oriented Eczema Measure; PPNRS, Peak Pruritus Numeric Rating Scale; PVRS, Pruritus Verbal Rating Scale; SCORAD, SCORing Atopic Dermatitis; SD-VAS, Sleep Disturbance Visual Analog Scale; TC, topical corticosteroids; URTI, upper respiratory tract infection; WPAI-AD, Work Productivity and Activity Impairment-Atopic Dermatitis.
In the first study in humans with a single dose administered during phase 1/1b, nemolizumab was associated with good tolerability and a significant reduction in pruritus of around 50% at week 4.13 In this study, which comprised 3 phases, the authors assessed doses ranging from 0.003mg to 3mg/kg in healthy adults with the following objectives: (a) to escalate the dose and evaluate safety and tolerability (first phase) with 0.3mg, 1mg, and 3mg/kg in healthy persons compared with placebo; (b) to evaluate the safety and tolerability of the doses chosen (second phase); and (c) to run a third phase that included the analysis of efficacy at the same doses in patients with AD compared with placebo. In this phase, the reduction in pruritus was accompanied by a significant improvement in sleep quality (around 80%) and a significant reduction in the use of hydrocortisone compared with placebo.13
A phase 2 study evaluated the safety and efficacy profile of nemolizumab at 0.1, 0.5, and 2mg/kg every 4 weeks, as well as at an exploratory dose of 2mg/kg every 8 weeks, both in the short term (12 weeks)33 compared with placebo and in the long term (extension of 52 weeks and 64 weeks in total).34 At 12 weeks, the authors reported a significant reduction in pruritus of approximately 60% (−43.7% with 0.1mg/kg, −59.8% with 0.5mg/kg, and −63.1% with 2mg/kg every 4 weeks and −20.9% with placebo; P<.05), which increased to approximately 80% at 64 weeks (−73% with 0.1mg/kg, −89.6% with 0.5mg/kg, and −74.7% with 2mg/kg every 4 weeks and −79% with 2mg/kg every 8 weeks). A rapid decrease in the visual analog scale (VAS) score for itch was observed from the first week of administration onward (the graphs separated clearly from the third day, with an average decrease in the 3 doses of 20% vs. 5% with placebo). The mean percentage change since onset in the Eczema Area and Severity Index (EASI), SCORing Atopic Dermatitis (SCORAD) tool, body surface area (BSA) affected, sleep disturbance, and Investigator Global Assessment (IGA) scale maintained the increase in values recorded from 12 to 64 weeks. EASI75 was reached at 64 weeks by approximately 70% of patients with nemolizumab (68%, 68%, 66% at 0.1, 0.5, and 2mg/kg every 4 weeks, respectively, and 74% at 2mg/kg every 8 weeks). This improvement corresponded to a reduction in the use of topical corticosteroids that was proportional to the dose administered.34
In order to facilitate clinical practice, a single flat dose was assessed independently of weight. A simulation based on the pharmacokinetic and pharmacodynamic analysis supported the use of a single monthly dose of between 25 and 50mg every 4 weeks for patients weighing less than 110 and 135kg. The simulated pruritus VAS supported the use of this dose optimization approach during subsequent phases of the clinical development program of nemolizumab.39
In their phase 2b trial, Silverberg et al.35 analyzed the effectiveness and safety of 10, 30, and 90mg of nemolizumab administered every 4 weeks compared with placebo, both in combination with topical corticosteroids for 24 weeks. A significant improvement in EASI was recorded for all the doses, with the 30-mg dose being the most effective. Nemolizumab 30mg every 4 weeks reduced EASI by −66.8% vs. 43.1% at 16 weeks and −68.8% vs. 52% every 24 weeks (P=.016 and P<.01 for nemolizumab and placebo, respectively). Significant reductions in EASI were recorded from week 8 (P≤.01). EASI50 was achieved at week 16 by 59.6% vs. 36.8% of patients (nemolizumab vs. placebo; P=.016) and EASI75 by 45.6% vs. 26.3% of patients (nemolizumab vs. placebo; P=.014). EASI50 was reached at 24 weeks by 66.7% vs. 43.9% of patients (vs. placebo; P=.014) and EASI75 by 45.6% vs. 26.3% (vs. placebo; P=.014). Nemolizumab improved itch (−67.3% vs. −35.8% with placebo at week 24), thus revealing significant differences with placebo from the first week of administration (P<.01). Improved findings were recorded for the IGA, Peak Pruritus Numerical Rating Scale (PPNRSR), Sleep Disturbance NRS, Dermatitis Life Quality Index (DLQI), EuroQoL 5-Dimension (EQ5D) score, and Hospital and Anxiety and Depression Scale (HADS), and the use of topical corticosteroids was reduced by 30%–40% vs. placebo.35 The 30-mg dose is currently being evaluated in several phase 3 pivotal trials. The results of these studies will provide substantial information on clinical dosing of nemolizumab.
Results have been published for a phase 3 trial based on 60mg every 4 weeks compared with placebo, both with topical corticosteroids (ie, as close to clinical practice as possible) and a 16-week follow-up. The primary objective was the reduction in the pruritus VAS score. Pruritus had diminished significantly at 16 weeks (nemolizumab vs. placebo, −42.8% vs. −21.42%; P<.001), as had EASI (−49% vs. −33.2%), DLQI (40% vs. 21%), and the Insomnia Severity Index (55% vs. 21%). Pruritus was recorded daily during the first week, thus making it possible to detect significant differences in the mean VAS score from day 2 after injection of nemolizumab.
In a meta-analysis of the safety and efficacy profile of nemolizumab in AD,40 nemolizumab led to significant improvements in safety and efficacy according to several clinical indices for AD, a significant decrease in EASI compared with placebo, and a significant reduction in the SCORAD value in the nemolizumab group compared with placebo. In the nemolizumab group, the pruritus VAS was −3.95 (95% CI, −5.56 to −2.37; P<.001). A significant decrease in the mean BSA score was observed for nemolizumab compared with the control group (standardized mean difference [SMD]=−0.19; 95% CI, −0.35 to −0.03; P=.019), as well as in the findings for EASI (SMD=−0.31; 95% CI, −0.45 to −0.17; P<.001) and IGA (RR, 0.81; 95% CI, −0.65 to −1.01; P=.064).40
Nemolizumab has been shown to be highly efficacious for relief of pruritus.13,33–38 In addition, it acts quickly from the first dose, with differences already observable from the second day after injection37 in the form of a rapid improvement in the EASI (significant from week 8 onward35). A recent study measured the speed at which systemic drugs used to treat AD reduce pruritus.41 The time to achieve a meaningful clinical response for itch reduction (TIMEACLIR-Itch) was calculated. Nemolizumab reached the TIMEACLIR-Itch quicker than biologics targeting IL-4 or IL-4/13, with a speed that was similar to that of small molecule inhibitors (JAK), thus demonstrating the role of IL-31 in causing pruritus and the role of JAK inhibitors in improving it.41
These data seem to clarify the role of IL-31 as the cause of itch, inflammation, and impairment of the skin barrier, as well as skin remodeling. Nemolizumab breaks the itch-scratch cycle, and its rapid effect on itch translates immediately into improved sleep quality, improved dermatitis (EASI and SCORAD), and improved quality of life (DLQI). This rapid relief from itch and improved sleep quality were associated with increased workplace productivity and reduced limitations in daily activities.36 Patients in a phase 2 study33,34 responded to the Work Productivity and Activity Impairment-Atopic Dermatitis (WPAI-AD) questionnaire. Nemolizumab improved productivity and activity significantly from week 4, with this improvement maintained until week 64. The improvement in the WPAI-AD was directly correlated with the magnitude of the improvement in pruritus and sleep quality.36 The severity of AD and quality of life affect the WPAI-AD, albeit to a lesser extent, thus highlighting that rapid control of pruritus and sleep quality should be the main target in the management of affected patients.
Nemolizumab at 0.5mg/kg every 4 weeks was compared with placebo in moderate and severe PN in a 12-week phase 2 study.38 The primary variable was the percent change from baseline in the average maximum score for pruritus on the NRS at week 4. The secondary outcomes included additional measures of itch and disease severity up to 12 weeks. The initial pruritus score was 8.4 in each group. At week 4, pruritus diminished by −4.5 points (−53.0%) in the nemolizumab group, as compared with −1.7 points (−20.2%) in the placebo group; P<.001 (the change in pruritus in favor of nemolizumab had already manifested from the second day after injection, −10.3% vs. −4.4%). Of the patients receiving nemolizumab, 29% reported no or almost no itch compared with 0% in the placebo group. As for PN lesions, 75% of lesions were cured with nemolizumab in 24% of patients compared with 11% in the placebo group. Sleep quality, as evaluated using the Sleep Quality NRS and comparing nemolizumab with placebo, improved from as early as the first week (−23.4% vs. −5.1%) and had improved further at 4 weeks (−56.4% vs. −26.6). Pruritus values remained unchanged at 12 weeks compared with 4 weeks, and the mean number of lesions decreased with nemolizumab (−12.6 vs. −6.1). Quality of life, defined as a reduction of at least four points in the DLQI (minimum clinically important difference) improved compared with placebo (59% vs. 31%).
Nemolizumab: Tolerability and SafetyThe tolerability and safety of nemolizumab were evaluated in the abovementioned studies and meta-analyses. Nemolizumab generally had a good safety and tolerability profile that was similar to that of placebo in studies on AD in the short term (12, 16, and 24 weeks)13,33,35,37 and was maintained in the long term (64 weeks).34
Treatment-related adverse effects included worsening of AD and peripheral edema (worsening of AD was not correlated with effectiveness for reducing pruritus, and the underlying mechanisms are not clear), abnormal creatine kinase values, nondermatologic infections (nasopharyngitis, upper respiratory tract infection, and gastroenteritis), and injection site reaction (this became less frequent with continued use of the product).34
In a study on PN,38 the safety profile of nemolizumab was similar to that of placebo. The adverse effects associated with treatment were nasopharyngitis, upper respiratory tract infection, digestive symptoms (abdominal pain and diarrhea), and musculoskeletal disorders (arthralgia, back pain, fibromyalgia, and jaw pain).
ConclusionsThe cytokine IL-31 is a key regulator of multiple mechanisms in chronic inflammatory skin diseases such as AD and PN. Given its essential role in the direct stimulation of sensory neurons associated with pruritus and its contribution to inflammation and remodeling of the impaired skin barrier, IL-31 connects the immune system in the skin with the nervous system.
The mechanism of action of nemolizumab enables it to modulate the neuroimmune response, directly blocking itch signaling and rapidly alleviating sensory itch. Nemolizumab also makes it possible to control inflammation and reduce the severity of eczema in AD and of the pruriginous lesions in PN by restoring epithelial function and promoting the integrity of the skin barrier.
Clinical trials with nemolizumab clearly show that blocking IL-31 leads to a more marked reduction in pruritus than placebo over 64 weeks in patients with moderate or severe AD who had not achieved a suitable response to topical drugs and antihistamines. Improvements were also observed in the variables associated with these disorders and measured using instruments such as EASI, SCORAD, DLQI, and IGA.
Nemolizumab is a new alternative that will provide dermatologists with the disease control they need to transform both the daytime and nighttime of patients with AD and PN.
FundingDrafting of this review was supported by a research grant from Galderma.
Conflicts of InterestThe authors declare that they have no conflicts of interest.