Psoriatic arthritis is a psoriasis-related spondyloarthropathy that occurs in 20–30% of patients with psoriasis. Various imaging studies have demonstrated that there is a considerable proportion of undiagnosed psoriatic arthritis among patients with psoriasis. Since early detection and treatment of psoriatic arthritis could, ultimately, allow the prevention of clinical and radiologic progression of the disease, there is the need to establish clinical indicators to detect this risk.
Nail psoriasis has been proposed as a predictor for the development of psoriatic arthritis. The inflammation involving the entheses, called enthesitis, is an early inflammatory change seen in psoriatic arthritis, and nail changes appear to result from the close relationship between the nail and the enthesis of the distal interphalangeal extensor tendon, one of the main entheseal compartments affected in psoriatic arthritis.
As skin lesions precede articular symptoms in more than 75–80% of patients with psoriatic arthritis, dermatologists may play a key role in the early detection and management of psoriatic arthritis.
La artritis psoriásica es una espondiloartropatía relacionada con la psoriasis que aparece en un 20–30% de los pacientes con psoriasis. Varios estudios por imágenes han demostrado que hay una cantidad considerable de artritis psoriásica no diagnosticada entre los pacientes con psoriasis. Existe la necesidad de establecer indicadores clínicos que señalen el riesgo de desarrollo de artritis psoriásica, ya que la detección y el tratamiento temprano de la misma podría, en última instancia, permitir la prevención y la progresión clínica y radiológica de la enfermedad.
Se ha propuesto la psoriasis ungueal como factor predictivo del desarrollo de la artritis psoriásica. La entesitis, inflamación de la entesis, es un cambio inflamatorio temprano observado en la artritis psoriásica, y los cambios en las uñas parecen ser el resultado de la estrecha relación entre la uña y la entesis interfalángica distal del tendón extensor, que es uno de los principales compartimentos entésicos afectados en la artritis psoriásica.
Los dermatólogos pueden desempeñar un papel clave en la detección temprana y en el manejo de la artritis psoriásica, ya que en más de un 75–80% de los pacientes con artritis psoriásica las lesiones de la piel preceden a la aparición de los síntomas articulares.
Psoriasis is a chronic, systemic, inflammatory disorder, affecting 2–3% of the population worldwide.1 Psoriatic arthritis (PsA) is a psoriasis-related spondyloarthropathy that presents with typical signs and symptoms of both psoriasis and arthritis and, like psoriasis, follows a chronic course.2-4 An estimated 20–30% of psoriasis patients may develop PsA.5,6 Imaging studies have demonstrated the existence of considerable number of patients with psoriasis and undiagnosed PsA, a reflection of the presence of subclinical arthritic disease in clinically normal joints.7,8
Persistent joint inflammation can lead to bone damage, and it is estimated that half of PsA patients develop irreversible joint lesions within the first few years of disease.9,10 Therefore, PsA is a severe, erosive and deforming condition.2
PsA patients have an increased burden of disease, impairment in quality of life, and diminished functional capacity, all reflected in a lower general health state.4,11,12 Overall, this results in great physical, psychological, and ultimately economic burden of the disease to the individual and society.13 There is therefore a need to establish a clinical indicator to detect risk and ensure early diagnosis of PsA. Early detection and treatment of PsA could, ultimately, allow the prevention of clinical and radiologic progression of the disease. For this reason, predictors for the presence of subclinical PsA are of considerable clinical interest. If validated properly, such indicators may help identify patients with subclinical disease at risk of deterioration, and allow an early intervention.2,4
Nail changes are observed in about 40% of psoriasis patients, a percentage that increasers to about 80% in patients with PsA. Nail disease in psoriasis has long been proposed as a predictor for the development of PsA.14–17
Since skin lesions precede articular symptoms in more than 75–80% of patients with PsA, with a mean estimated delay of 10 years, there is a potential window of opportunity for the early diagnosis and management PsA.2,4,18 This represents a unique occasion to document the clinical changes predictive of the development of PsA, or its subclinical presence.19 As dermatologists usually see patients with psoriasis before arthritis develops, they are in a unique position to diagnose PsA in its earliest phase, by detecting the precocious silent alterations of the disease even before radiological signs and symptoms have become manifest.20,21 The ultimate goal is early detection and appropriate treatment, avoiding disease progression and irreversible bone damage.
Psoriatic arthritis: clinical findingsPsA is a seronegative spondyloarthropathy, whose central defining feature is inflammation involving the entheses (enthesitis). PsA often presents in a characteristically asymmetrical manner, commonly involving the distal joints of the hands and feet. This specific distal joint affection points toward the presence of some factors, intrinsic to the target joint itself, which act as key drivers in the onset and perpetuation of the disease process.20,22,23
A common defining feature of PsA is the clinical presence of dactylitis, which represents inflammatory involvement (often with diffuse swelling) of the distal interphalangeal (DIP) joint. The DIP joint involvement begins as inflammation of the entheses, the main change in PsA. This perpetuated, chronic inflammatory process may ultimately culminate in joint cavity involvement, with osteolysis and periarticular new bone formation.23
With the purpose of creating a uniform and established definition of PsA, a Classification Criteria for Psoriatic Arthritis – the CASPAR classification – was introduced. This classification is found to be highly specific for the diagnosis of PsA (98.7%), and easier to use than other existing classification criteria. In this classification, diagnosis of PsA is supported by the combined presence of a certain number of clinical features, such as: (a) established inflammatory joint disease; (b) current psoriasis; (c) history of psoriasis; (d) family history of psoriasis; (e) dactylitis; (f) radiographic evidence of juxta-articular new bone formation; (g) negative rheumatoid factor, and (h) typical psoriatic nail dystrophy.24 As suggested by these criteria, nail disease in PsA is given a prominent role in diagnosis, and is given an equal footing to other important clinical and radiographic criteria.
Entheses: the anatomical site of joint inflammationEnthesis is the term used for the attachment site of ligaments, tendons, and joint capsules to bone. This anatomical structure appears to share microanatomical features with the skin, both assisting in the resilience to regional compressive and shear force applications, helping preserve tissue homeostasis.25
As mentioned earlier, several imaging and histological studies have defined enthesitis (inflammation of the entheses) as the central, early inflammatory change in PsA.20,26,27
With the aim of characterizing arthritis in PsA patients, several investigators studied the DIP joint with imaging techniques such as ultrasonography and magnetic resonance imaging (MRI). The extent of the enthesis-associated disease in PsA became evident, as these techniques allowed to detect subclinical imaging features of inflammation of the entheses in clinically normal joints. Furthermore, these entheseal changes were found to be consistently identified when clinically evident nail disease was present.7,22,27,28 These imaging studies not only sustained the now established notion that enthesopathy is the major feature of PsA, but also suggested that nail disease in psoriasis may be associated with subclinical entheseal disease.
The entheses appear to derive their nourishment from the adjacent synovium, reflecting the anatomical proximity these structures.22 As such, inflammation and consequently altered entheseal function affects not only fibrous fibers in the entheses but the synovium as well and, by consequence, the contents of the synovial compartment, the joint surfaces.
As mentioned above, like the skin, the entheses are the anatomical structures that respond to both shear and compressive forces, and is now considered a prominent target of the early inflammatory process in PsA. As in the skin, changes observed in the nail-entheseal-joint apparatus could be explained by a Koebner response phenomenon. This hypothesis defines the occurrence of a common Koebnerization phenomenon – the appearance of lesions at previous sites of microdamage and trauma – and ultimately, inflammatory changes resulting from this stress could be responsible for the development of both nail disease and PsA joint changes.4,29 This concept of joint Koebnerization can ultimately be considered an aberrant response to mechanical stress.4,25,30
The link between psoriasis and HLA-Cw6 is well established, the latter representing the strongest genetic risk factor for the development of psoriasis.31 Nonetheless, joint and nail psoriasis-related changes appear to lack this association. Currently, studies so far have attested the presence of a prominent innate inflammatory infiltrate in PsA joints. This helped emphasize the emerging notion that, unlike psoriasis (in which an autoimmune response phenomenon, whose main participants are innate, but most importantly the adaptive immunity), PsA results from a Koebnerization phenomenon triggering an auto-inflammatory reaction of neutrophils in a tissue prone to stress lesions. In conclusion, the adaptive immune response that is presumed to be related to skin disease may not play a prominent role in PsA.22
Nail apparatus relation to arthritis: the entheseal complexOf all the clinical indicators studied so far in the prediction of the development of PsA, the most strongly associated has been undoubtedly nail disease.4 An incidence study that followed a cohort of 1593 psoriatic patients for 30 years concluded that, compared to subjects without nail disease, psoriatic patients with nail dystrophy were almost 3 times more likely to develop PsA, with an attributed hazard ratio (HR) of 2.93 (95% CI, 1.68–5.12).19 Additionally, a retrospective analysis of 4146 psoriatic patients pointed to nail involvement as the strongest predictor for concomitant PsA, with an odds ratio (OR) of 2.93 (95% CI, 2.51–3.42).4 In addition, a prevalence study that aimed to determine the clinical implications of nail disease in 661 psoriasis patients found an association between nail changes and PsA, with an OR of 3.25 (95% CI, 2.16–4.90).32
The nail unit is formed by: (1) the nail plate; (2) proximal nail fold; (3) matrix; (4) nail bed and (5) hyponychium. The resulting specific nail lesion differs according to the nail structure primarily affected by the inflammatory process.23
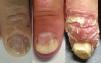
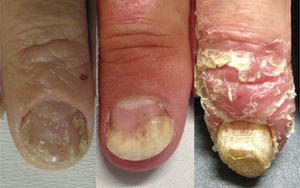
Thus, the characteristics of nail involvement are determined by the extension and site of the inflammatory reaction. If the nail matrix is involved, there may be development of pitting, leukonychia, red patches (erythema) in the lunula, onychorrhexis, and onychodystrophy. In contrast, if the nail bed is affected, oil spots, splinter hemorrhage, onycholysis and subungual hyperkeratosis may develop.4,23,30,33 Although various studies differ in terms of the most prevalent nail change detected in PsA patients, pitting and onycholysis are defined as the most common modifications (Fig. 1).33
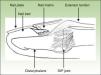
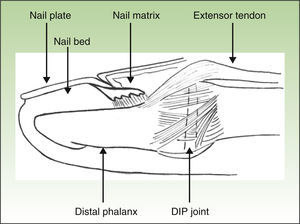
Anatomical and imaging studies have made a substantial contribution to our current understanding of the nail unit and its intrinsic connections to the DIP joint. These studies provided an anatomical link between the DIP extensor tendon enthesitis and the nail changes in PsA (Fig. 2).26
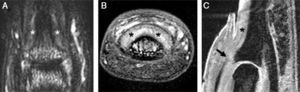
Magnetic resonance imaging of a distal interphalangeal (DIP) joint of a 29-year-old female with a 3-year history of psoriatic arthritis affecting the joint: (A) T2-weighted fat-suppressed coronal image of the dorsal DIP joint showing high signal around the joint and adjacent to the distal phalanx near the nail bed (denoted by asterisk). (B) T1-weighted fat-suppressed post-gadolinium axial image of the same joint showing enhancement and thickening of the tissues under the nail bed (denoted by asterisk). (C) Water-selective excitation sagittal sequence of the joint demonstrating the thickened tissues under the nail bed (denoted by asterisk) and an abnormal extensor tendon enthesis, which was thickened and showed loss of the low signal (arrow). Linear regions with low signal similar to the extensor tendon could be seen enveloping the nail (arrowheads). These may correspond to fibers from the extensor tendon extending toward the nail bed.
The nail is just as much an integral part of the entheseal unit as it is of the skin.4,26 At the microanatomical level, a close relationship exists between the nail and the DIP extensor tendon enthesis. As represented schematically in Fig. 3, the DIP extensor tendon attaches distally to the DIP joint, to a region located on the dorsal surface of the distal phalanx (DP). Arising from this attachment site, fibrous connections link nail structures, namely the nail matrix, to the periosteum of the DP.22 The DIP joint is therefore linked to the nail structures via the entheseal unit of the DIP extensor tendon.26,34
Recognition of this joint-entheseal-nail apparatus highlights the importance of entheseal inflammatory changes in PsA. This close structural relationship helps us understand why PsA patients, who typically present enthesitis of the DIP joint, concurrently develop inflammatory nail changes.4
As noted above, clinically unrecognized enthesitis is commonly observed in early PsA and, at this stage, crude radiographic signs are usually absent.30
In patients with psoriasis, imaging indicators of joint inflammation were found more frequently in patients with nail disease in comparison with those who presented no nail dystrophy signs. For example, the presence of extensor tendon entheseal thickening by ultrasound was observed in 42% of psoriatic patients (35/83) with clinical nail changes, while only 17.4% of patients (15/86) without nail lesions were found to have ultrasonographic findings. Entheseal changes are therefore more frequently observed in patients with nail changes, highlighting the relationship between nail and entheseal inflammation.20,27
In a study that evaluated the nail and DIP joint in patients with PsA using MRI, it was observed that nail involvement was present in almost all PsA patients (95.7%), even when the presence of clinical onychodystrophy was not evident.28 These findings suggest that nail disease is virtually always present in PsA patients, although not always clinically obvious. Patients could therefore benefit from close follow-up and evaluation by an expert in the nail area.
Furthermore, this group found that inflammatory signs of DP involvement always overlapped with nail involvement and cases of DIP joint changes alone were reported (that is, without nail and DP inflammatory changes). This enabled the formulation of a theory in which DIP joint involvement may arise as a result of nail and DP affection.28 In conclusion, the clinical and imaging findings of this study suggested psoriatic nail changes and distal phalanx inflammatory involvement precede DIP joint changes. These observations prompted the idea that nail dystrophy in psoriatic patients could be an indicator of ongoing inflammatory involvement of the distal phalanx, the site of attachment of entheseal structures of the DIP extensor tendon.28 Other studies corroborated this theory, providing strong evidence that nail disease is a predictor of PsA before arthritic changes occur.35,36
For this reason, nail disease in psoriasis may represent an accessible and readily observable indicator of future inflammatory joint affection. It could therefore be used as a sensitive clinical predictor of PsA.
Nail assessment: the dermatology–rheumatology gapStudies have shown that, when assessing psoriatic nail disease, imaging findings of nail changes correlate well with clinical nail assessments.27
Studies that assessed PsA severity did not observe a direct relation between the degree of nail affection and DIP joint inflammation.37 From this assessment, one can conclude that the smallest nail changes, which may go unnoticed to the untrained observer with the naked eye, could be an important indicator of the presence of major disease. Expert examination, particularly dermatologists who can readily detect such changes, is therefore important. In this setting, the dermatological examination is of prime importance as a detector of a silent disease manifestation requiring referral (in this case, to the rheumatologist).
In a comparison between nail change detection, about 15% of patients classified by rheumatologists as having clinically evident nail disease, had another specific nail diagnosis unrelated to psoriasis, such as onychomycosis or onychoschizia, when observed by dermatologists.16 This underscores another essential characteristic of the dermatological assessment – high specificity.
In this view, psoriatic nail disease represents an area of overlap between dermatology and rheumatology. Mutual awareness of this overlap by dermatologists and rheumatologists, along with referral of psoriatic patients deemed to be at risk for arthritic disease, is of particular importance for the prevention of a serious, mutilating, chronic disease.38
Dermatologists can, therefore, play a central role in the early detection and management of PsA.
ConclusionSpecific imaging techniques have shown that the central inflammatory change in PsA takes place in the entheseal compartment. This structure is present in virtually every joint, but enthesitis manifests clinically in PsA especially in those structures subject to major shear and stretch forces.
Nail disease in PsA results from the close relationship between this structure and the enthesis of the DIP extensor tendon – one of the main entheseal compartments affected in PsA. The inflammatory change begins in the entheses, affecting the nail according to the degree and site (matrix vs nail bed) of inflammatory activity, and progresses proximally to affect the DIP joint. This results ultimately in the final anatomical, radiological and clinical changes of PsA in the joints.
In this view, the ability to detect nail changes by dermatologists gives them a strategic role in the early detection of subclinical entheseal disease and in the referral and management of early PsA, thereby preventing severe, erosive and deforming joint lesions.
In conclusion, the dermatological assessment in psoriatic patients represents a unique opportunity to prevent serious and functionally limiting disorders, thereby benefiting the health system in general and, most importantly, improving the patient's quality of life.
Ethical responsibilitiesProtecting people and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data privacyThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors declare no conflict of interests.
Dr Ai Lyn Tan and Oxford University Press, on behalf of the British Society for Rheumatology, for their permission for Fig. 2.