New onset of autoimmune blistering skin diseases may be impacted by several factors, such as drugs, viruses, or vaccines. Isolated cases may be induced by SARS-CoV-2 vaccination.1 We report an unusual case of mucous membrane pemphigoid (MMP) triggered by SARS-CoV-2 vaccine.
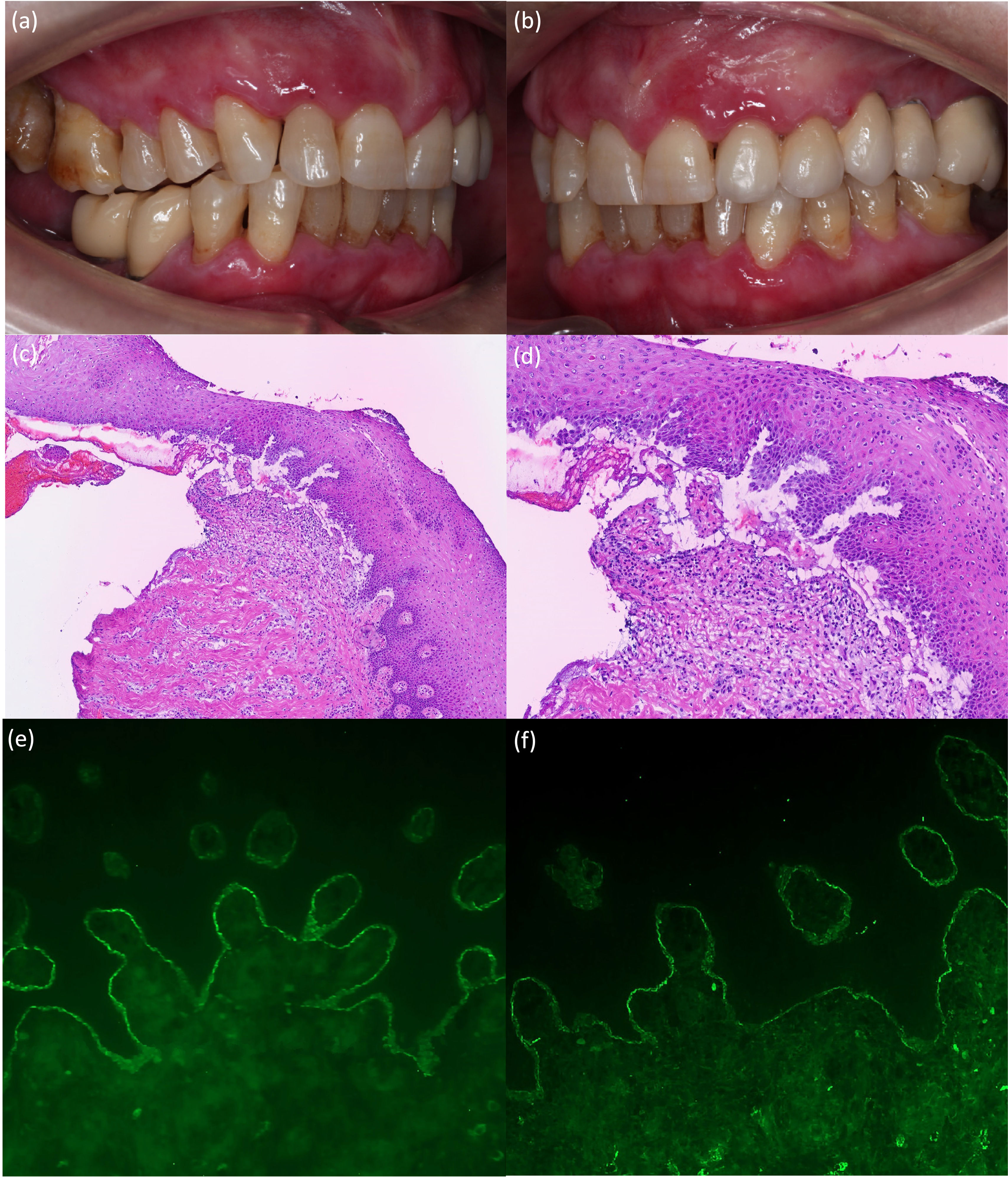
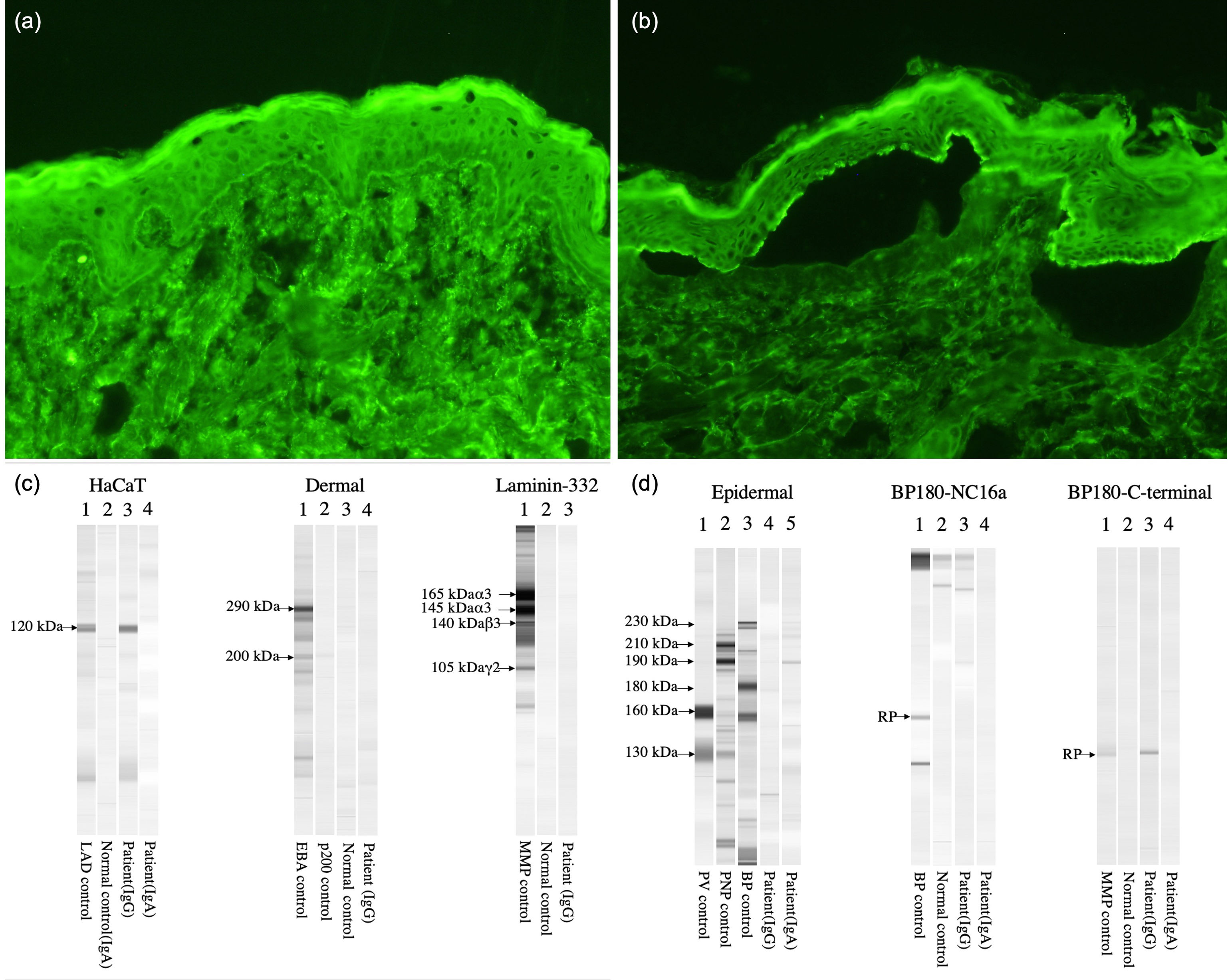
A 75-year-old-woman was evaluated for painful oral mucosal lesions. No personal dermatological diseases were reported. The patient came with a 12-month history of lesions she noticed 1 week after having received the 2nd dose of the Pfizer SARS-CoV-2 vaccine. Previous treatment with topical corticosteroids had been unsuccessful. Physical examination revealed the presence of erosive and erythematous plaques on the upper and lower gingivae (Fig. 1a, b). Neither the skin nor the other mucosal surfaces were affected. The oral mucosal biopsy performed revealed the presence of subepidermal detachment and inflammatory cells including eosinophils (Fig. 1c, d). Direct immunofluorescence showed linear depositions of IgG and C3 along the epidermal basement membrane zone (Fig. 1e, f). Moreover, using the salt-split procedure (IIF-SS) linear IgG deposits (1:40) where seen on the epidermal side (Fig. 2a, b). An ELISA test performed on the patient's serum detected a high level of anti-BP-180 antibodies (116U/mL; normal<20U/mL). By immunoblotting assay, IgG against the C-terminal and LAD-1 domains of BP180 were found (Fig. 2c, d). Our patient was diagnosed with anti-BP180-type MMP, probably induced by the SARS-CoV-2 vaccine. Dapsone 50mg/12h and topical clobetasol propionate were initiated, leading to lesion improvement until complete remission was achieved 4 months later.
(a, b) Erosive and erythematous plaques with a diameter of up to 1cm affecting the marginal, interdental, and attached gingiva. (c, d) Representative histologic image from the biopsy showing a subepidermal blister and multiple inflammatory cells including eosinophils (hematoxylin and eosin, ×100 (c) and ×200 (d)). (e, f) IFD test showing (e) linear deposition of IgG (×200) and (f) C3 along the dermoepidermal junction (×200).
(a, b) Indirect immunofluorescence images. (a) Patient's IgG react on basement membrane zone, 1:10 dilution (×200). (b) Patient's IgG react on epidermal side of the section, 1:40 dilution (×200). (c, d) Immunoblotting assay showing IgG vs the C-terminal and LAD-1 domains (HaCaT) of BP180. Results tested negative for epidermal (desmoglein 1 and 3) and dermal extracts, BP180 NC16a, and Laminin-332.
Globally, several cases of autoimmune disorders, including autoimmune bullous diseases (AIBDs), have reportedly been developed after SARS-CoV-2 vaccination.2 Currently, only 6% of patients with AIBDs after SARS-CoV-2 vaccines developed de novo AIBDs. The reported AIBDs after the administration of the SARS-CoV-2 vaccine are bullous pemphigoid, linear IgA disease, pemphigus vulgaris, MMP and pemphigus foliaceus.1 In most cases, vesicular and bullous eruptions flared up after the administration of the 1st and/or 2nd doses.
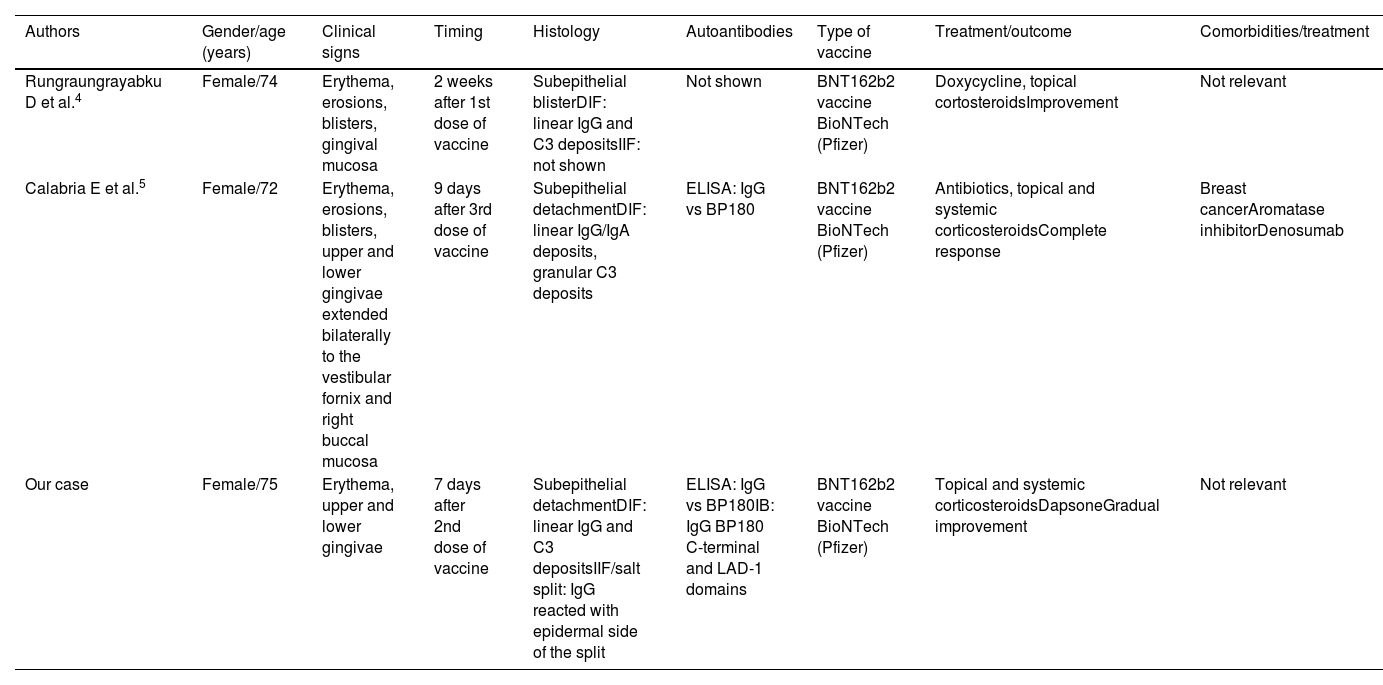
The development of autoimmune bullous oral lesions after SARS-CoV-2 vaccination has been infrequently reported in literature and usually in association with skin lesions. There is a slight prevalence for women (69%), and the mean time of onset following vaccination is 9.4 days. The BNT162b2 BioNTech vaccine (Pfizer) tends to trigger autoimmune oral lesions more frequently.3 Only 2 cases of MMP after SARS-CoV-2 vaccination have been reported4,5 (Table 1). All these patients provide interesting information. First, MMP after SARS-CoV-2 vaccination occurred mainly in women, same as conventional MMP. The 3 patients developed exclusively oral mucosal lesions and 2 patients exhibited IgG autoantibodies vs BP180 detected by ELISA. In addition, our case developed IgG autoantibodies vs LAD-1 and the C-terminal domains of BP180 as shown by immunoblotting assays. In our case, IIF-SS showed IgG reactivity with the epidermal side. Finally, all 3 MMP cases induced by SARS-CoV-2 vaccine had an excellent prognosis after treatment, probably due to the self-limited effect associated with the vaccine. All these findings indicate that SARS-CoV-2 vaccination could trigger MMP.
Cases reported of mucous membrane pemphigoid after COVID vaccine.
| Authors | Gender/age (years) | Clinical signs | Timing | Histology | Autoantibodies | Type of vaccine | Treatment/outcome | Comorbidities/treatment |
|---|---|---|---|---|---|---|---|---|
| Rungraungrayabku D et al.4 | Female/74 | Erythema, erosions, blisters, gingival mucosa | 2 weeks after 1st dose of vaccine | Subepithelial blisterDIF: linear IgG and C3 depositsIIF: not shown | Not shown | BNT162b2 vaccine BioNTech (Pfizer) | Doxycycline, topical cortosteroidsImprovement | Not relevant |
| Calabria E et al.5 | Female/72 | Erythema, erosions, blisters, upper and lower gingivae extended bilaterally to the vestibular fornix and right buccal mucosa | 9 days after 3rd dose of vaccine | Subepithelial detachmentDIF: linear IgG/IgA deposits, granular C3 deposits | ELISA: IgG vs BP180 | BNT162b2 vaccine BioNTech (Pfizer) | Antibiotics, topical and systemic corticosteroidsComplete response | Breast cancerAromatase inhibitorDenosumab |
| Our case | Female/75 | Erythema, upper and lower gingivae | 7 days after 2nd dose of vaccine | Subepithelial detachmentDIF: linear IgG and C3 depositsIIF/salt split: IgG reacted with epidermal side of the split | ELISA: IgG vs BP180IB: IgG BP180 C-terminal and LAD-1 domains | BNT162b2 vaccine BioNTech (Pfizer) | Topical and systemic corticosteroidsDapsoneGradual improvement | Not relevant |
A cause–effect relationship between SARS-CoV-2 vaccine and autoimmunity has not been completely established to this date. Several hypotheses have been postulated as an explanation for the new onset or flare-ups of AIBD following SARS-CoV-2 vaccination. These theories include molecular mimicry between the virus and human proteins, bystander activation, anti-idiotypic networks, and epitope spreading.2 Moreover, vaccine adjuvants may enhance an immune response. Of note, SARS-CoV-2 vaccines generate spike proteins, which may bind to the angiotensin-converting enzyme-2 receptors on keratinocytes, thus leading to the recruitment of CD4+ lymphocytes. Nevertheless, a recent meta-analysis of autoimmune skin disorders after SARS-CoV-2 vaccination shows that they are not associated with a higher risk than other triggering factors,6 meaning that it should be recommended in patients who need protection vs SARS-CoV-2 infection.
Conflict of interestThe authors declare that they have no conflict of interest.