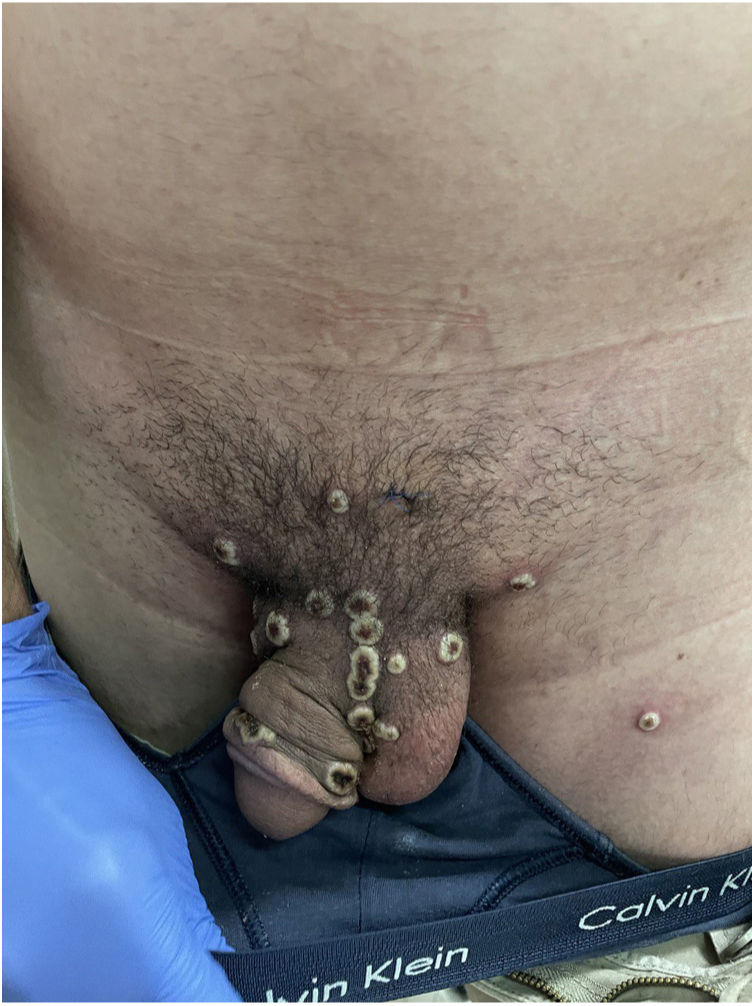
A 32-year-old man attended the Sandoval Health Center in the Madrid region, Spain, because of some disseminated lesions that had appeared 8 days earlier (Fig. 1). He identified as a man who has sex with men (MSM). His personal history included HIV infection, controlled with highly active antiretroviral therapy, with undetectable viral load, and past gonococcal urethritis infection with Chlamydia trachomatis.
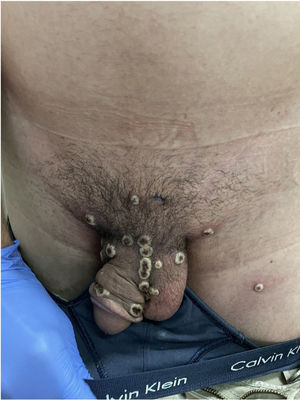
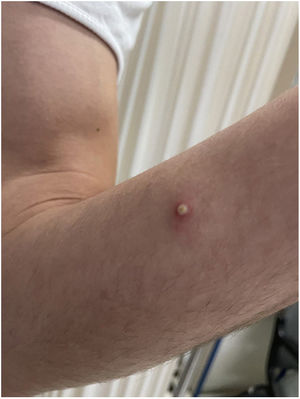
The patient explained that, after the appearance of the lesions on the genitals, arms, and palms, he attended a health clinic where, empirically and without diagnosis, he was prescribed treatment with doxycycline and prednisone for approximately one week. He did not remember the dose. At the same time, imiquimod 5% cream was applied for 5 days, on the suggestion of an acquaintance due to suspicion of infection by molluscum contagiosum (Fig. 2).
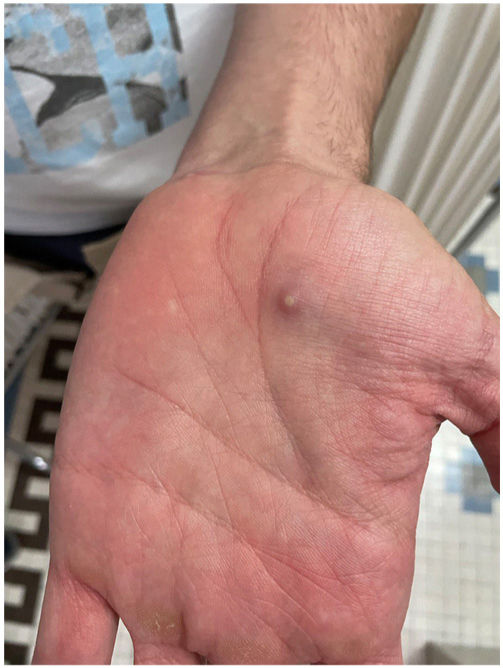
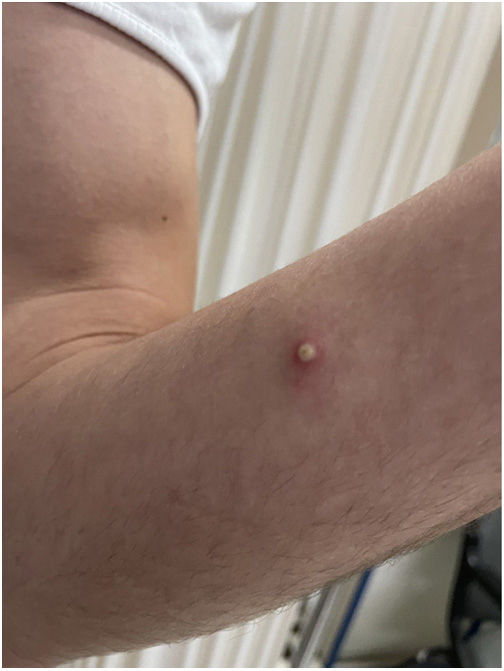
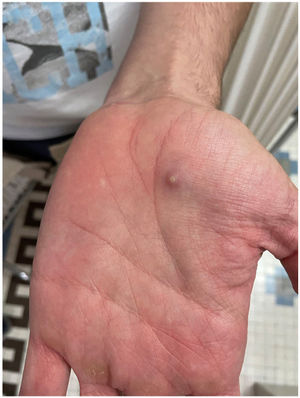
The examination revealed multiple umbilicated pustules with a dark center on the genitals and pubis, both arms, and the palms of the hands with peripustular inflammation. He also presented with painful bilateral swollen inguinal lymph nodes (Fig. 3).
Faced with suspected infection by the monkeypox virus, the patient was questioned again. He mentioned having been in a sauna, where he maintained sexual relations with men, 10 days before onset of fever and general malaise. Two days later, he experienced an outbreak of pustular lesions.
To confirm infection with the monkeypox virus and to rule out other sexually transmitted diseases, rectal exudate, blood, and urine samples, and samples from the lesions were taken to perform the polymerase chain reaction (PCR) test for Orthopoxvirus in the National Microbiology Center in Majadahonda, Madrid, Spain.
The patient, in accordance with public health recommendations, self-isolated at home for approximately 10 days, during which time he received oral support therapy with anti-inflammatory and antipyretic agents, as well as a solution of sulfated water for the skin lesions.
The PCR results were positive for the monkeypox virus and the patient progressed satisfactorily with slow resolution of the lesions. Complete clearance occurred 2 weeks after the start of isolation.
The case described is one of the first confirmed cases of the outbreak of monkeypox that started in Madrid during May 2022.1 The monkeypox virus is transmitted to humans by contact with infected animals or humans or through material contaminated with the virus. The virus penetrates the organism through solutions of continuity in the skin, respiratory tract, or mucus membranes. The incubation period is between 6 and 16 days, although it can range from 5 to 21 days. There is evidence of asymptomatic monkeypox infections in individuals vaccinated against smallpox and in unvaccinated individuals.2
It is assumed that the main factor for monkeypox infection in humans is viral transmission through direct or indirect contact with living or dead animals. This can occur through bites, direct contact with fluids or lesions of an infected animal or contaminated material. Another possible source of contagion is eating inappropriately cooked meat of an infected animal.
Human–human transmission is rare, but outbreaks with such transmission have been reported. In addition, monkeypox can be transmitted by direct contact with body fluids of an infected person or contaminated objects such as clothing and bed linen.
The capacity for detection of monkeypox virus DNA by PCR of samples from suspected skin lesions has been well established.
Given that viremia is short-lasting, it is preferable to obtain samples from exudate, scabs, or aspirate of fluid from lesions instead of blood samples. Real-time PCR can discriminate not only between monkeypox virus and other Orthopoxvirus, but also between different strains of the monkeypox virus itself.3
Serology is of limited value given immunological cross-reactivity with other Orthopoxvirus pathogens in humans. However, it might be useful to rule out recent infection by Orthopoxvirus for contact tracing and populational serological study. Immunohistochemistry may be used to identify antigens in biopsies and to rule out or identify other suspected infectious agents.4
There are no vaccines or specific treatment available for monkeypox. The disease is managed by treating symptoms and providing support measures, including prevention and treatment of bacterial superinfections. Currently, there are no specific vaccines for monkeypox. First and second-generation smallpox vaccines administered during the smallpox eradication programs to all those born before March 1978 provide effective protection against the monkeypox vaccine.
Given their adverse effects, these vaccines have generally not been used for control of the monkeypox virus. However, in the United States, 30 individuals were vaccinated against smallpox in 2003 during that outbreak. There is a third generation of vaccines (MVA-BN/Imvanex) against smallpox developed for those individuals for whom the previous versions of the vaccine are contraindicated. This vaccine is derived from a virus that has lost its ability to replicate in primate cells.
The vaccine has a much better safety profile than previous generations of smallpox vaccines. It is approved in Europe for vaccination against smallpox in adults and was used in the United Kingdom in 2018 after cases of monkeypox were identified.
Public health measures aim to reduce human to human transmission through early recognition of cases, based on clinical suspicion and laboratory tests, isolation of infected patients, implementation of the appropriate infection prevention and control measures in health centers (standard, contact, aerosols), and early detection of possible new cases by contact tracing and investigation of sites of outbreaks.
The smallpox vaccine can be offered to contacts, including healthcare workers in contact with patients, individuals exposed to the monkeypox virus at sites of outbreaks, and to partners and other close contacts.
The protective effect of the human smallpox vaccine has been demonstrated in clinical trials in the 1980s, with efficacy greater than 85%.5
The World Health Organization (WHO) suggests that national health authorities should offer the smallpox vaccine to those who treat patients or have been exposed to patients or samples therefrom.
According to the Centers for Disease Control in the United States, early vaccination with the smallpox vaccine, that is, within 14 days after exposure, is an option to consider to reduce symptoms of the monkeypox virus.
Since the worldwide eradication of smallpox, the vaccine for this disease is not available to the general public, but several countries and the WHO maintain it in stock. Smallpox vaccines manufactured with old technologies should not be administered to immunocompromised individuals.6
Conflict of interestsThe authors declare that they have no conflict of interest.