Until a few years ago, severe cases of atopic dermatitis (AD) were treated with corticosteroids and classical-immunosuppressors. However, these treatments are not suitable for long-term treatment due to safety concerns specially in children and adolescents. The approval of biological therapies and JAK-inhibitors to be used in adolescents with moderate-to-severe AD has expanded therapeutic options.1 Upadacitinib has been added to the therapeutic armamentarium of adolescents (≥12 years) with AD and the stepped care plan for this group in the latest version of the European clinical practice guidelines on the management of atopic eczema.2 Studies reporting real-life experiences with upadacitinib in adult patients with AD have been published3–6 however, evidence for its use in adolescents is scarce.7–10 This study aimed to assess the mid-term safety and efficacy profile of upadacitinib in adolescents (12–18 years) with moderate-to-severe AD in the real-world clinical practice.
We conducted a retrospective non-interventional multicentre study involving 16 Spanish hospitals from January 2022 through 2023. Disease severity was measured using the Eczema Area and Severity Index (EASI), the validated Investigator Global Assessment (IGA), and the pruritus Numerical Rating Scale (NRS). The primary endpoint was to analyze upadacitinib effectiveness in terms of absolute-EASI scores at weeks 4, 16 and 24 (when available), as well as the percentage of patients achieving an EASI-75 and EASI-90 response. Secondary endpoints included assessing IGA, pruritus NRS, and the drug safety profile. Statistical analysis included measures of central tendency and dispersion for continuous variables, and frequencies for categorical variables. Logistic regression assessed the impact of weight, disease duration, gender, and asthma on EASI response.
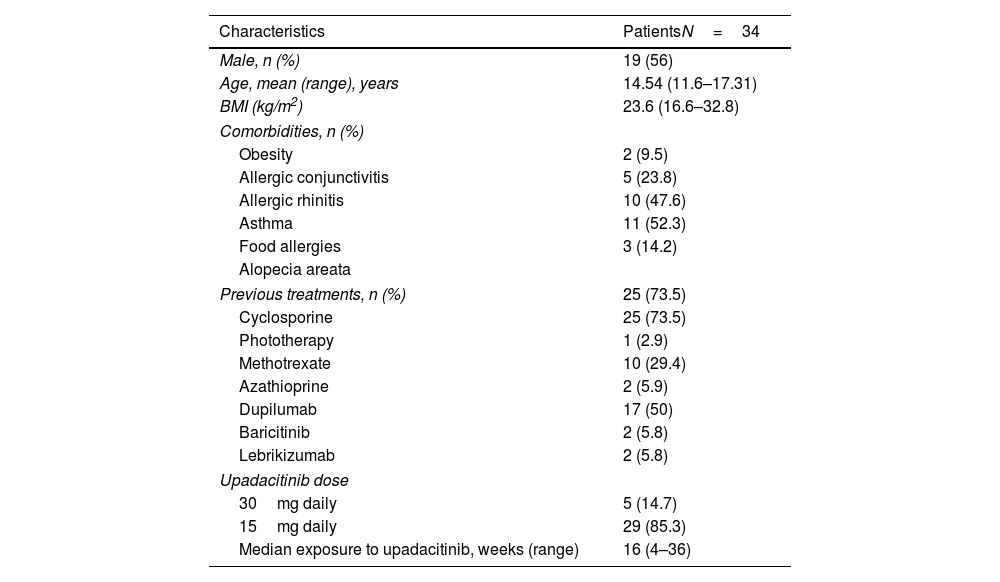
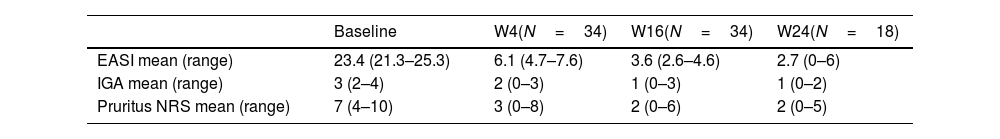
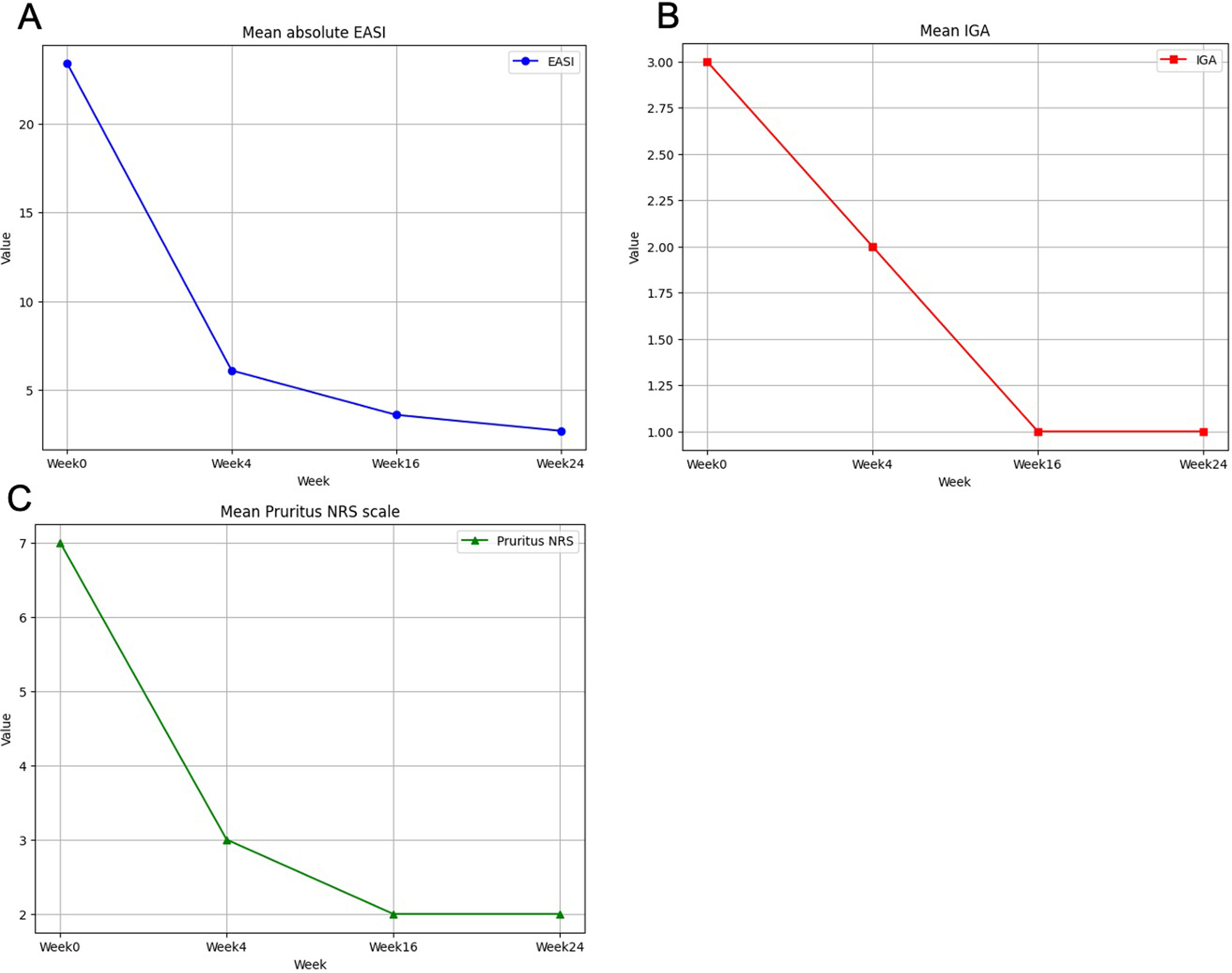
A total of 34 patients were included. Demographic and clinical characteristics are shown in Table 1. The mean disease duration was 6.3 (0.4–15) years. The median BMI was 22.5 (16.6–32.8) and 6 (17.6%) patients were obese (BMI >30kg/m2). The most common concomitant atopic comorbidities were asthma (23.5%) and allergic rhinitis (20.6%). Approximately 73.5% of the patients had previously received systemic therapies prior to upadacitinib onset (73.5%, cyclosporine and 50%, dupilumab). The occasional use of low-potency topical-corticosteroids was allowed. Upadacitinib was the first systemic immunomodulator (naïve) for 9 patients. Most patients (85.3%) received a daily dose of upadacitinib of 15mg. Rapid response to upadacitinib was observed. The median (range) EASI score was 23.4 (confidence interval 95%, 21.3–25.3) at baseline dropping down to 6.1 (4.7–7.6), 3.6 (2.6–4.6) and 2.7 (0–6) on weeks 4, 16 and 24 respectively. A rapid decrease in the NRS itching score occurred, dropping from a median of 7 points at baseline down to 3 points at week 4 (Table 2 and Fig. 1 of the supplementary data). Already at an early stage (week 4), 26 and 20 patients achieved EASI-75 and EASI-90 responses, respectively. A statistically significant relationship was not found across the variables gender, weight, duration of atopic dermatitis, or presence of asthma and probability of achieving an EASI-90 response (supplementary table). In patients previously on dupilumab and baricitinib, the drug effectiveness was as good as in “bio-naïve” patients. Three patients did not achieve an EASI-75 response throughout the evaluated period. Severe infections or major adverse cardiovascular events (MACE) were not reported. Up to 4 patients reported a mild worsening of acne which was treated with topical products, while 3 patients experienced a worsening of their AD.
Demographic and baseline clinical characteristics.
| Characteristics | PatientsN=34 |
|---|---|
| Male, n (%) | 19 (56) |
| Age, mean (range), years | 14.54 (11.6–17.31) |
| BMI (kg/m2) | 23.6 (16.6–32.8) |
| Comorbidities, n (%) | |
| Obesity | 2 (9.5) |
| Allergic conjunctivitis | 5 (23.8) |
| Allergic rhinitis | 10 (47.6) |
| Asthma | 11 (52.3) |
| Food allergies | 3 (14.2) |
| Alopecia areata | |
| Previous treatments, n (%) | 25 (73.5) |
| Cyclosporine | 25 (73.5) |
| Phototherapy | 1 (2.9) |
| Methotrexate | 10 (29.4) |
| Azathioprine | 2 (5.9) |
| Dupilumab | 17 (50) |
| Baricitinib | 2 (5.8) |
| Lebrikizumab | 2 (5.8) |
| Upadacitinib dose | |
| 30mg daily | 5 (14.7) |
| 15mg daily | 29 (85.3) |
| Median exposure to upadacitinib, weeks (range) | 16 (4–36) |
Upadacitinib effectiveness throughout the study.
| Baseline | W4(N=34) | W16(N=34) | W24(N=18) | |
|---|---|---|---|---|
| EASI mean (range) | 23.4 (21.3–25.3) | 6.1 (4.7–7.6) | 3.6 (2.6–4.6) | 2.7 (0–6) |
| IGA mean (range) | 3 (2–4) | 2 (0–3) | 1 (0–3) | 1 (0–2) |
| Pruritus NRS mean (range) | 7 (4–10) | 3 (0–8) | 2 (0–6) | 2 (0–5) |
EASI: Eczema Area and Severity Index; IGA: Investigator Global Assessment; SD: standard deviation; NRS: Numerical Rating Scale; W: week. Calculated for a 95% CI (confidence interval).
This study demonstrates the rapid and sustained response of upadacitinib in the treatment of moderate-to-severe AD in adolescents in the real-world setting. Patients had severe disease profiles and were refractory to multiple systemic therapies, including biological treatments and other JAK inhibitors (baricitinib) which differs from those in clinical trials.1 An interesting finding is that most of our subjects treated with either 30mg or 15mg daily achieved the EASI-75 and EASI-90 responses very early (76.4% and 58.8% respectively) on week 4. Our results showed higher EASI-75 response rates on week 16 vs clinical trials of AD adolescents on upadacitinib, which is consistent with findings reported by Hagino et al.8 That rapid response in adolescent has also been reported and confirmed by other authors.7–10 Patients experienced a quick reduction in pruritus NRS a few days after starting treatment, even greater than that obtained in the trials of upadacitinib.1 The limitations of the study include its observational and retrospective design, small sample size, and short follow-up. In conclusion, as far as we know, this is one of the largest real-world clinical series to date, with a significant number of adolescent patients on upadacitinib reporting on the short-term efficacy and rapid response of upadacitinib in achieving itch control even in long-standing recalcitrant cases and those who discontinued treatment with dupilumab due to loss of efficacy or side-effects.
CRediT authorship contribution statementF.J. Melgosa Ramos and Sergio Santos Alarcón have equally contributed to the conception, design, data collection, data analysis, interpretation, writing – draft preparation and review of the present work. The final version to be published has been approved by all the authors. The rest of the authors have contributed to the conception design, data collection and review of the present work.
Ethics statementThe study 59 was conducted in accordance with the principles contained in the Declaration of Helsinki for studies involving humans. All patients (and parents) provided their informed written consent.
FundingAuthors have not received any sources of funding (institutional, private and corporate financial support) for the work reported in this paper.
Conflict of interestThe author(s) declare(s) that there are no conflicts of interest regarding the publication of this paper.
AcknowledgementsTo Antonio Torrelo Fernández, Raúl de Lucas Laguna, Salvador Arias Santiago, Almudena Mateu Puchades, Altea Esteve Martínez, Carlos Abril Pérez, Laia Curto Barredo, Minia Campos Domínguez, Juan José Pereyra-Rodriguez, Mónica Munuera Campos, Ana Martín Santiago, Gloria Garnacho Saucedo, Eulalia Baselga Torres, Juan Francisco Silvestre Salvador, Lucero Noguera Morel, Esther Fiz Benito, José Mª Sánchez Motilla, Violeta Zaragoza Ninet, Mercedes Rodríguez Serna, Ana Llull Ramos and Victor González Delgado for their contribution on data collection.
Data availabilityAll data are available under request from the authors.