Merkel cell carcinoma, though rare, is one of the most aggressive tumors a dermatologist faces. More than a third of patients with this diagnosis die from the disease. Numerous researchers have attempted to identify clinical and pathologic predictors to guide prognosis, but their studies have produced inconsistent results. Because the incidence of Merkel cell carcinoma is low and it appears in patients of advanced age, prospective studies have not been done and no clear treatment algorithm has been developed. This review aims to provide an exhaustive, up-to-date account of Merkel cell carcinoma for the dermatologist. We describe prognostic factors and the imaging techniques that are most appropriate for evaluating disease spread. We also discuss current debates on treating Merkel cell carcinoma.
El carcinoma de células de Merkel es un tumor muy poco frecuente, pero es uno de los más agresivos a los que se puede enfrentar un dermatólogo. Más de un tercio de los pacientes fallece por esta enfermedad. Numerosos investigadores han intentado identificar los posibles factores clínico-patológicos relacionados con el pronóstico de este carcinoma. Sin embargo, los resultados obtenidos en estos estudios son discordantes. Debido a la baja frecuencia y la edad avanzada de los pacientes, no se dispone de estudios prospectivos, y en consecuencia, no existe un claro algoritmo en el tratamiento. Este artículo pretende realizar una exhaustiva y comprensiva revisión del carcinoma de células de Merkel que suponga al dermatólogo una puesta al día en este tumor. Detallamos los factores pronósticos, se revisan las técnicas de imagen que resultan más adecuadas para el estudio de extensión y las controversias actuales relacionadas con el tratamiento.
Merkel cell carcinoma (MCC) is a rare, highly aggressive tumor, and local or regional disease recurrence is common, as is metastasis. Because of the low incidence of this tumor and the advanced age of patients, prospective studies comparing treatment protocols for different stages have not been done. At present we lack consensus on how to manage the treatment of MCC once diagnosed.
This review aims to provide an exhaustive, up-to-date account of MCC for the dermatologist. We describe prognostic factors and the imaging techniques that are most appropriate for evaluating disease spread. We also discuss current debates on how to treat MCC.
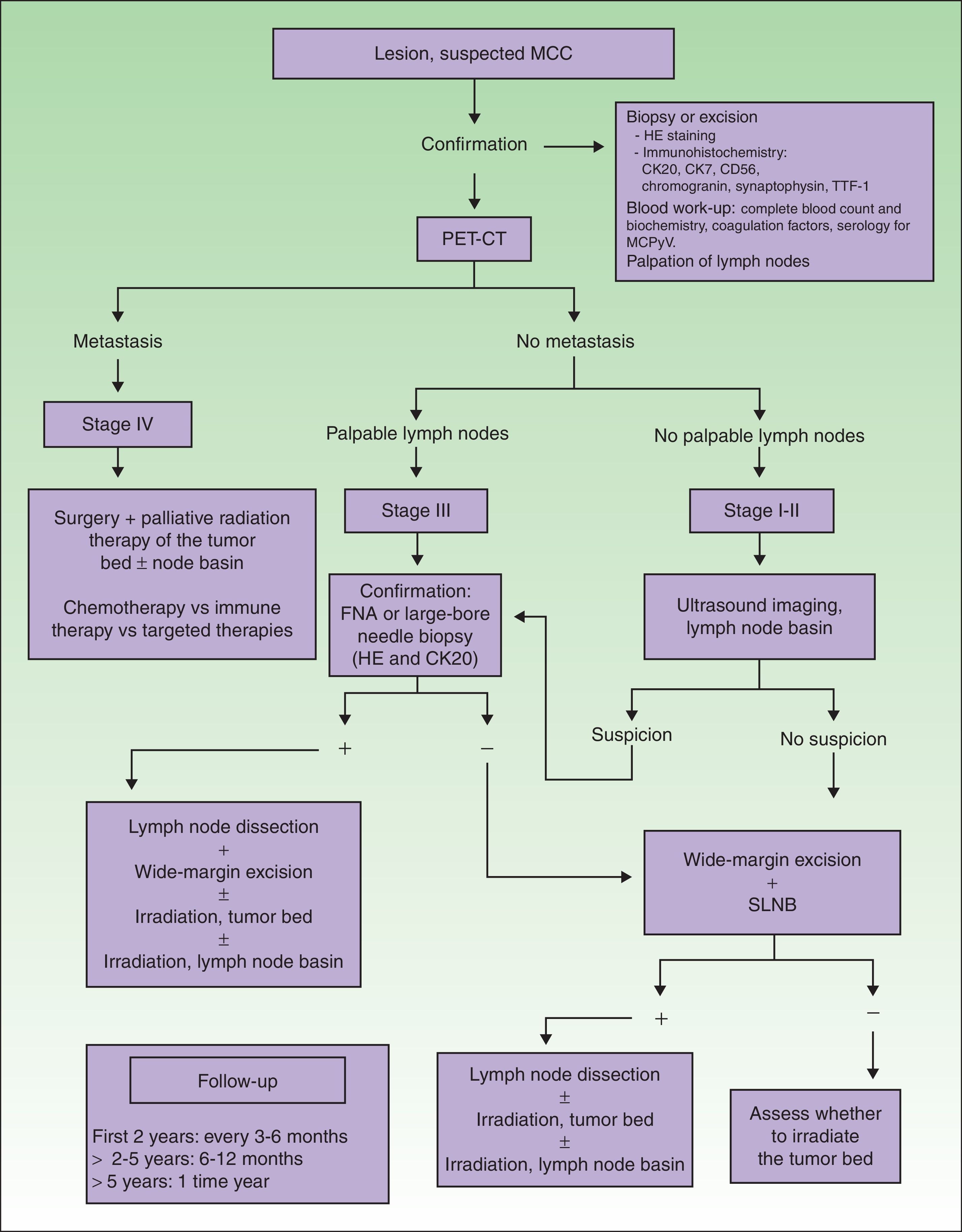
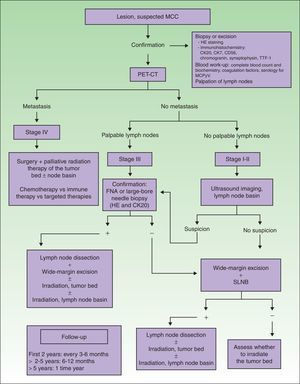
Imaging Studies of MCC ExtensionNo clinical management guidelines reflecting consensus on the most appropriate test batteries and imaging studies to establish MCC tumor extension and guide follow-up have emerged.1
The entire surface of the patient's skin must be examined and regional lymph nodes palpated to detect evidence of spread.1
An exhaustive blood workup including a full blood count and biochemistry for alkaline phosphatases and coagulation factors should be done.2 A baseline serum test for the Merkel cell polyomavirus (MCPyV) should be ordered if possible. High antibody titers are specific indicators of recent disease and changes in blood levels reflect response to treatment; thus, increases are considered markers of recurrence.3
An imaging study must be obtained for initial staging in order to rule out distant metastasis. Computed tomography (CT) and magnetic resonance imaging are usually recommended.4 New generation positron emission (PET) CT provides simultaneous capture of images of metabolic activity and the anatomical location of lesions5–7 (Fig. 1). This information is of great importance because it can affect staging: Concannon et al.6 found that stage classification changed in 33% of patients based on fluorodeoxyglucose PET-CT and that the approach to management changed in 43%.
Diagnostic and treatment algorithm for MCC. MCC refers to Merkel cell carcinoma; CK, cytokeratin; HE, hematoxylin-eosin; TTF-1, thyroid transcription factor 1; MCPyV, Merkel cell polyomavirus; PET-CT, positron emission tomography–CT; SLNB, sentinel lymph node biopsy; and FNA, fine-needle aspiration.
When tumors appear localized on clinical examination, showing no evident sign of metastasis, it is important to firmly establish whether regional lymph nodes are involved or not (Fig. 1), given that nodal spread is associated with a worse prognosis.4
There is ample evidence of the usefulness of ultrasound imaging to explore spread to lymph nodes in melanoma. However, the use of this technique in MCC is still limited. Zageretal.8 proposed ultrasound imaging to study the lymph node basins draining MCCs in patients at high surgical risk who cannot undergo sentinel lymph node biopsy (SLNB) as well as to follow patients in whom node involvement is uncertain. Along that line, Righietal.9 recently suggested a protocol that combined ultrasound imaging with fine-needle aspiration as a step prior to SLNB in selected patients. When there are palpable nodes (stage III), this approach can confirm regional metastasis. In the absence of palpable lymph nodes (stages I and II), ultrasound exploration and fine-needle aspiration can be followed by cytology and immunohistochemistry (Fig. 1) to detect cells positive for cytokeratin (CK) 20.1 Patients with positive results of cytology are referred for lymph node dissection. This approach circumvents SLNB in at-risk patients, and those in whom nodal spread is not suspected based on ultrasound imaging are not referred for SLNB. The sensitivity of this approach was 85.7% and specificity was 90% in the study of Righi and colleagues.
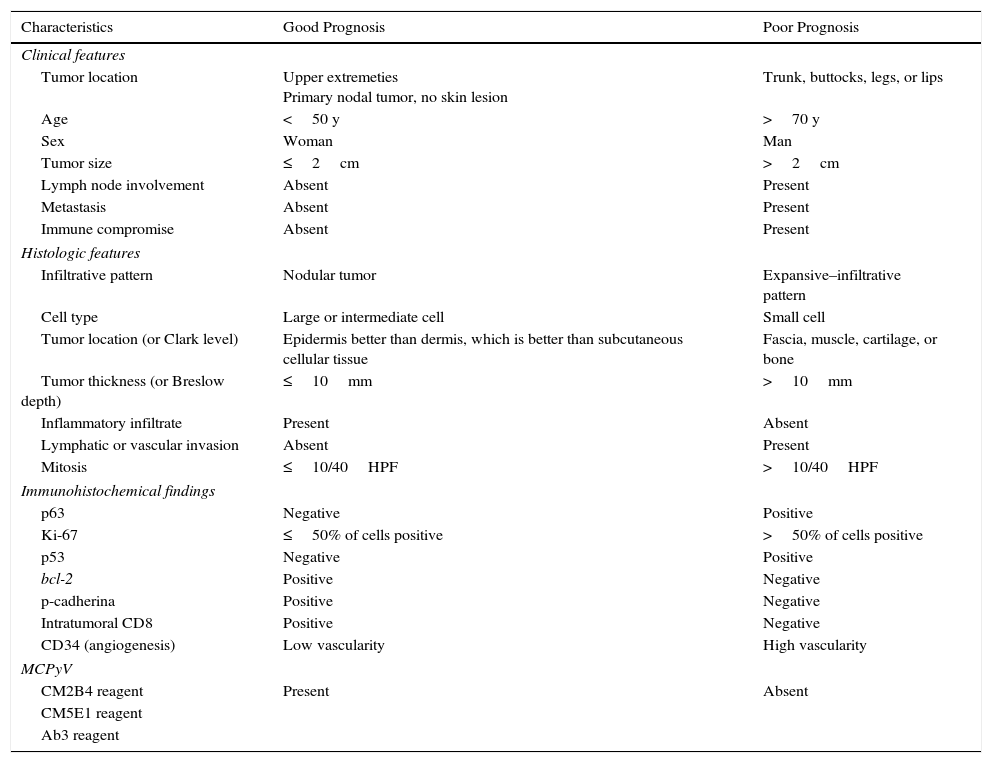
Prognostic FactorsMany have tried to identify factors that might affect prognosis in MCC, but studies have produced inconsistent results. The main clinical, histologic, and immunohistochemical indicators of prognosis are summarized in Table 1.10
Main Clinical, Histologic, and Immunohistochemical Characteristics Associated With Prognosis in MCC.
| Characteristics | Good Prognosis | Poor Prognosis |
|---|---|---|
| Clinical features | ||
| Tumor location | Upper extremeties Primary nodal tumor, no skin lesion | Trunk, buttocks, legs, or lips |
| Age | <50 y | >70 y |
| Sex | Woman | Man |
| Tumor size | ≤2cm | >2cm |
| Lymph node involvement | Absent | Present |
| Metastasis | Absent | Present |
| Immune compromise | Absent | Present |
| Histologic features | ||
| Infiltrative pattern | Nodular tumor | Expansive–infiltrative pattern |
| Cell type | Large or intermediate cell | Small cell |
| Tumor location (or Clark level) | Epidermis better than dermis, which is better than subcutaneous cellular tissue | Fascia, muscle, cartilage, or bone |
| Tumor thickness (or Breslow depth) | ≤10mm | >10mm |
| Inflammatory infiltrate | Present | Absent |
| Lymphatic or vascular invasion | Absent | Present |
| Mitosis | ≤10/40HPF | >10/40HPF |
| Immunohistochemical findings | ||
| p63 | Negative | Positive |
| Ki-67 | ≤50% of cells positive | >50% of cells positive |
| p53 | Negative | Positive |
| bcl-2 | Positive | Negative |
| p-cadherina | Positive | Negative |
| Intratumoral CD8 | Positive | Negative |
| CD34 (angiogenesis) | Low vascularity | High vascularity |
| MCPyV | ||
| CM2B4 reagent | Present | Absent |
| CM5E1 reagent | ||
| Ab3 reagent | ||
Abbreviations: bcl-2, B-cell lymphona 2 oncogene; HPF, high-power field; MCC, Merkel cell carcinoma; MCPyV, Merkel cell polyoma virus.
Most agree that overall survival in MCC depends mainly on stage at clinical diagnosis.11–14 In a study of 251 patients, Allenetal.15 reported an 81% survival rate for patients diagnosed in stage I (67% for stage II, 52% for stage III, and 11% for stage IV).
Reported clinical predictors of poor prognosis are advanced age (>70years)16; male sex17; immunocompromised status14; tumor size of more than 2cm on diagnosis18; and a tumor location on the trunk,13 buttocks, legs, or mucosal tissues.19 MCC may also start in the lymph nodes without a skin tumor. Such primary nodal tumors account for 8% to 12% of all MCCs and are associated with a better prognosis.20,21
The largest case series to analyze histologic factors was reported by Andeaetal.22 The factors they initially found to be related to a poor prognosis were tumor size, thickness, and depth; an infiltrative growth pattern; the presence of lymphatic and vascular invasion; and the absence of a peritumoral lymphocytic infiltrate. However, only an infiltrative growth pattern, lymphatic and vascular invasion, and deep extension of the tumor survived as predictors on multivariate analysis.
Lacking evidence from prospective studies, various authors have reported associations between certain immunohistochemical markers and MCC prognosis (Table 1). However, no immunohistochemical marker is widely used and recognized to have prognostic value at present. The Ki-67 protein is highly expressed in most MCC tumors. Fernández-Figuerasetal.23 found that MCCs that recur or metastasize have higher Ki-67 expression than those that do not.23 Others have reported an association between high Ki-67 expression and a shorter disease-free period after treatment and poorer prognosis.24,25
Asiolietal.25 reported that expression of p63, a member of the p53 family, was an independent predictor of shorter survival in MCC. They found that 53% of tumors had p63+ cells and that their course was more aggressive (P<.0001). More recently published studies described similar results.26,27 Therefore, although studies are few and based on few patients, the importance of the effect of p63 expression on survival suggests it is one of the most important prognostic factors in this disease.
The presence of MCPyV can be detected by microbiological or immunohistochemical methods. Touzéetal.28 found that patients with tumors containing the virus have a better prognosis and longer survival time. However, others have cast doubt on those results after finding that nearly all MCCs (97%) harbor the virus.29
TreatmentMCC is a very aggressive cancer with a high incidence of local and regional recurrence and distant metastasis. Unfortunately, a clear treatment algorithm for MCC is not available because of the low incidence of the disease and advanced age of the patients. Controlled trials to compare different therapeutic approaches, therefore, have not been done.
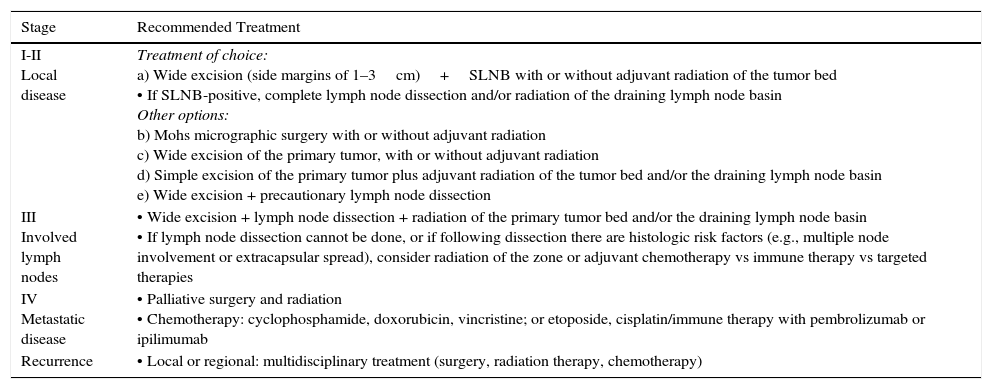
In spite of the current lack of a standard protocol, the initial treatment of patients with tumors that have not metastasized (stages I–III) should target the primary tumor and regional lymph nodes (Table 2, Fig. 1).30
Treatment Protocol for MCC According to Stage.
| Stage | Recommended Treatment |
|---|---|
| I-II Local disease | Treatment of choice: a) Wide excision (side margins of 1–3cm)+SLNB with or without adjuvant radiation of the tumor bed • If SLNB-positive, complete lymph node dissection and/or radiation of the draining lymph node basin Other options: b) Mohs micrographic surgery with or without adjuvant radiation c) Wide excision of the primary tumor, with or without adjuvant radiation d) Simple excision of the primary tumor plus adjuvant radiation of the tumor bed and/or the draining lymph node basin e) Wide excision + precautionary lymph node dissection |
| III Involved lymph nodes | • Wide excision + lymph node dissection + radiation of the primary tumor bed and/or the draining lymph node basin • If lymph node dissection cannot be done, or if following dissection there are histologic risk factors (e.g., multiple node involvement or extracapsular spread), consider radiation of the zone or adjuvant chemotherapy vs immune therapy vs targeted therapies |
| IV Metastatic disease | • Palliative surgery and radiation • Chemotherapy: cyclophosphamide, doxorubicin, vincristine; or etoposide, cisplatin/immune therapy with pembrolizumab or ipilimumab |
| Recurrence | • Local or regional: multidisciplinary treatment (surgery, radiation therapy, chemotherapy) |
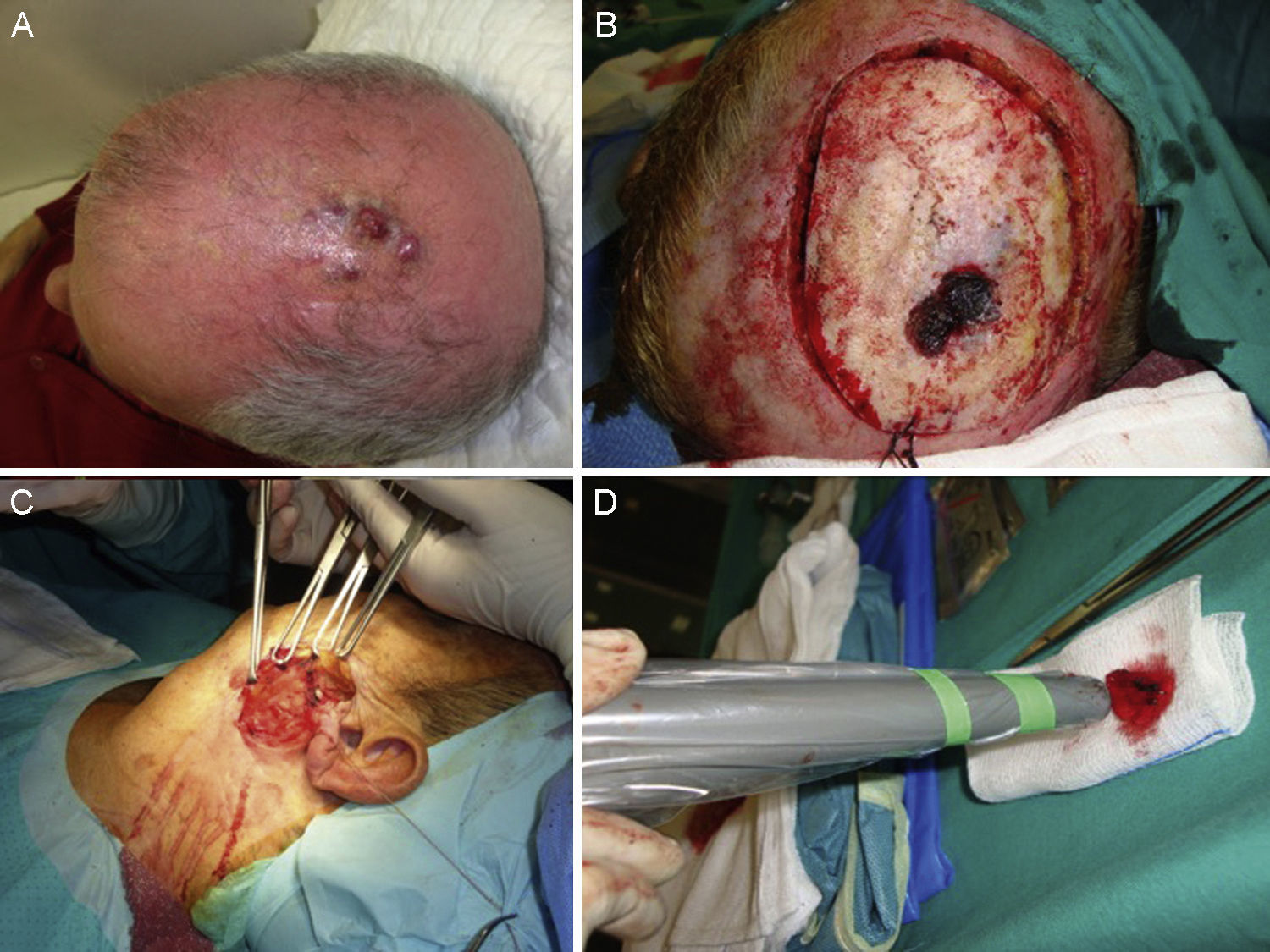
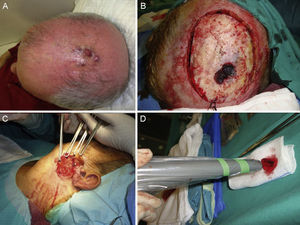
A certain level of agreement concerning surgical excision as the treatment of choice in MCC has formed. The principal aim of surgery is to excise enough to ensure tumor-free margins given that incomplete excision is associated with recurrence.12 No consensus has emerged as to the ideal size of side margins, however. Most of the oldest studies recommended margins of 2to 3cm, including the muscle fascia or galea (Fig. 2).31–34 A lower recurrence rate has been reported when margins of 1to 3cm have been targeted.33 However, such large margins are not always feasible, especially if the tumor is located on the head. Nor has increased overall survival been demonstrated to depend on larger margins. More recent studies recommend less generous margins. Tai12 proposed 1-cm margins for tumors measuring less than 2cm but 2-cm margins for larger tumors. The guidelines of the National Comprehensive Cancer Network (NCCN) recommends tighter margins of 1to 2cm, followed by adjuvant irradiation of the tumor bed.
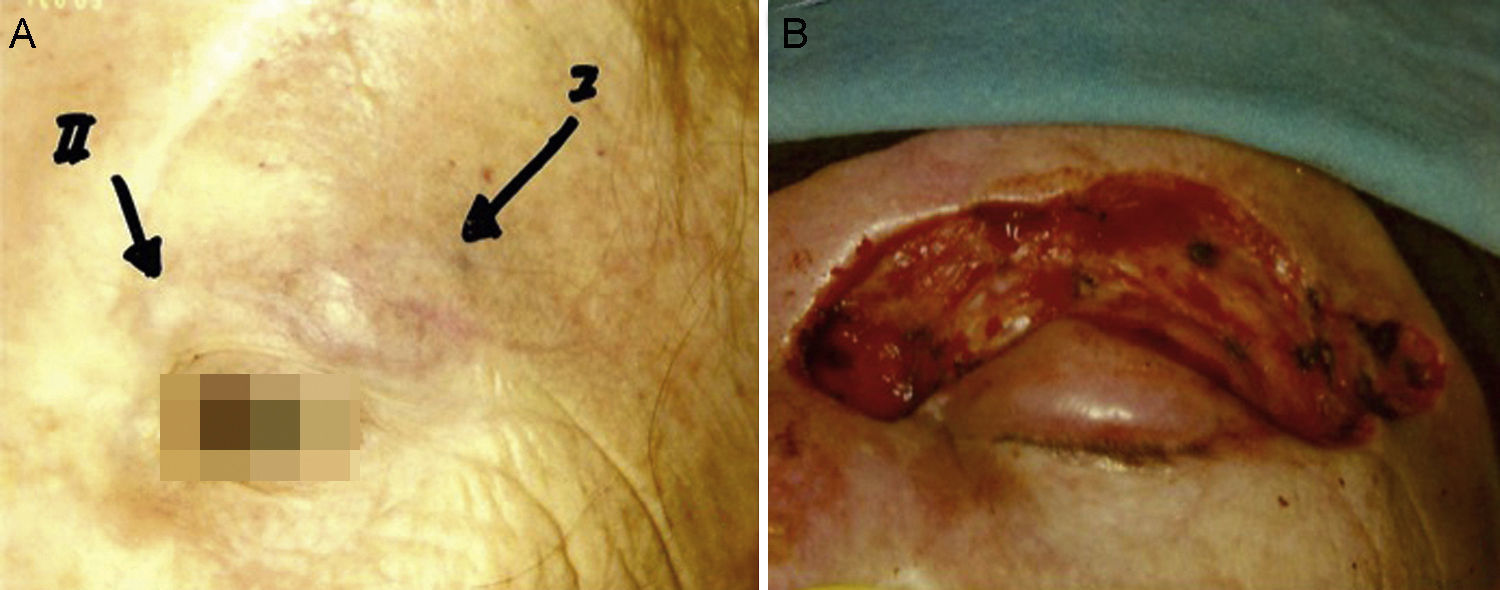
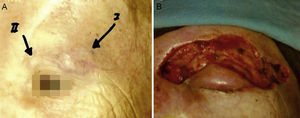
Some authors have proposed using Mohs micrographic surgery (Table 2), especially in areas where it is difficult to excise enough to obtain adequate margins, such as on the face and especially on the eyelid (Fig. 3).35,36 Boyeretal.37 studied a series of 45 cases treated with Mohs surgery followed or not by radiation, finding that a surgical margin of 1.67cm was necessary. If they had sought margins of 2or 3cm, 25% and 12% of the tumors, respectively, would have been inappropriately excised. Furthermore, nearly half the tumors required smaller margins of under 1cm, meaning that healthy tissue would have been removed unnecessarily if wider margins had been taken systematically. The experience of O’Connoretal.38 on using Mohs surgery in 12 patients was similar. Only 1 patient experienced local recurrence. In spite of such promising results, we find very few case series describing the treatment of MCCs with Mohs surgery.35–41 Therefore, we cannot say there is sufficient evidence to support a claim that this approach is more effective than traditional surgery with wider margins.
The frequency of local recurrence after surgery is high at between 27% and 32% when wide margins are taken, whereas frequencies as high as 70% to 89% have been reported in relation to smaller margins.42
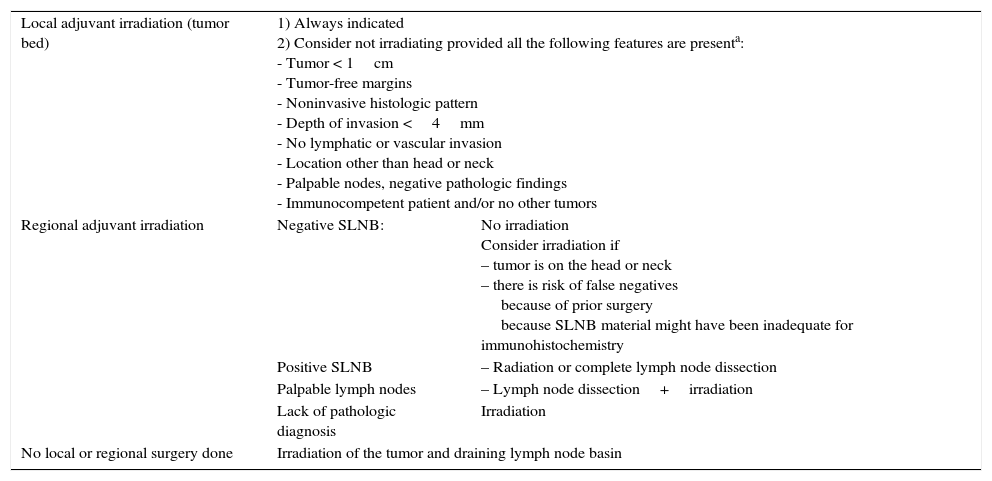
MCC is a highly radiosensitive tumor and in spite of the lack of randomized controlled trials43 because of the low incidence of cases, there is a considerable body of literature supporting the usefulness of postoperative radiation in reducing local recurrence. The problem of lack of randomized trials, however, means that debate continues42 (Table 3).
Indications for Local and Nodal Radiation Therapy in MCC.
| Local adjuvant irradiation (tumor bed) | 1) Always indicated 2) Consider not irradiating provided all the following features are presenta: - Tumor < 1cm - Tumor-free margins - Noninvasive histologic pattern - Depth of invasion <4mm - No lymphatic or vascular invasion - Location other than head or neck - Palpable nodes, negative pathologic findings - Immunocompetent patient and/or no other tumors | |
| Regional adjuvant irradiation | Negative SLNB: | No irradiation Consider irradiation if – tumor is on the head or neck – there is risk of false negatives because of prior surgery because SLNB material might have been inadequate for immunohistochemistry |
| Positive SLNB | – Radiation or complete lymph node dissection | |
| Palpable lymph nodes | – Lymph node dissection+irradiation | |
| Lack of pathologic diagnosis | Irradiation | |
| No local or regional surgery done | Irradiation of the tumor and draining lymph node basin | |
Abbreviations: MCC, Merkel cell carcinoma; SLNB, sentinel lymph node biopsy.
Lewis et al.44 found that local tumors were better controlled when surgery was followed by radiation (12% recurrence) than with surgery alone (39%) in a meta-analysis of 1254 cases from 132 studies. Similarly, Huietal.45 showed that age, tumor size, and local irradiation were associated with rates of local recurrence but that only postoperative radiation remained a predictor on multivariate analysis. Therefore, once MCC tumors are removed, many hospitals irradiate the surgical bed and a wide margin surrounding it in the interest of reducing recurrence and increasing survival. Not all experts agree on the routine use of radiation, however. Only 17% were irradiated in the series of 251 patients reported by Allen et al.,15 who saw no effect of radiation on local recurrence: 10% recurred after radiation therapy vs 8% without it. They recommended local irradiation be used only when tumor-free margins cannot be obtained and when histologic staging of lymph nodes has not been possible. Clarketal.46 similarly concluded that patients with tumors smaller than 1cm in diameter and negative lymph nodes can be followed without radiation if closely watched. The NCCN at present recommends radiation after excision of tumors regardless of stage although it does not benefit certain patients who are considered to be at low risk.47 The criteria for inclusion in this group of low-risk patients are shown in Table 3.
Boyer et al.37 observed marginal recurrence of MCC in 4% of patients in a group treated with a combination of Mohs surgery and postoperative radiation as opposed to 0% in the group treated with surgery alone, but the difference was not significant. Gollardetal.,41 in contrast, did observe benefits of adjuvant irradiation.
In patients of advanced age with many concurrent conditions that contraindicate surgery, radiation therapy alone is considered a possible and effective treatment. Mortieretal.48 described their experience treating 9 patients considered inoperable for medical reasons who had clinically negative nodes; they were irradiated as the only treatment at a mean dose of 60Gy. No recurrence was seen after 3 years’ follow-up. Veness and Richards49 treated 43 patients exclusively with radiation, achieving local control of the field in 75% and a 2-year survival rate of 58%.49
Macroscopic tumors are irradiated with a local dose of 60to 66Gy. For microscopically observed disease, the dose is 56to 60Gy, and if the surgical margins are negative, 50to 56Gy. The tumor is usually exposed to fractionated doses of 1.8to 2Gy 5 times per week.
Nodal BasinMCC metastasis to the lymph nodes occurs often and early, in around 30% of patients on average (range, 15% to 66%) at diagnosis,50,51 and in 79% over the course of disease.52 Approximately a third of MCC patients with clinically palpable but radiologically negative lymph nodes are found to have microscopically detectable lymphadenopathy.53 Because of these findings, an initial SLNB is recommended (Table 2, Fig. 1). Although SLNB in tumors smaller than 1cm was questioned for some time,54 most authors now opt to do the procedure regardless of size (Fig. 1). Thus, a retrospective study of 8044 MCCs found that a 14% risk of lymph node involvement in tumors measuring 0.5cm rose to 25% risk in tumors measuring 1.7cm and to 36% in tumors of more than 6cm.55 They also noted that the number of affected lymph nodes on diagnosis predicted survival (0 nodes, 76% 5-year survival; 1 node, 50%; 2 nodes, 47%; 3–5 nodes, 42%; ≥6 nodes, 24%). Biopsy results, therefore, could be useful for managing cases.
Because wide resection can alter the drainage of the primary tumor, SLNB should be done at the same time the tumor is excised, starting with the nodes.30
A 30% rate of false negatives in SLNB decreases to 22% when immunohistochemical analysis is used.56 Suetal.57 reported the highest diagnostic sensitivity and specificity for MCC micrometastasis to the lymph nodes was achieved when they used anti-CK20 antibody immunostaining.
A problem that emerges when managing an MCC tumor on the head or neck is the low predictability of the pattern of lymph drainage in these locations. According to some authors, drainage does not coincide with what is expected in 34% to 84% of these patients, increasing the risk of false negatives; furthermore bilateral drainage has been found in up to 10% of cases.58,59 In contrast, others have reported seeing unexpected drainage pathways in only 14% of cases.60 SLNB is technically more complex in the head and neck because it is more difficult to visualize lymphatic drainage by lymphoscintigraphy in this part of the body, where the potentially affected nodes are very close to the site of tracer injection. In addition, SLNB in the head and neck is associated with more complications because the nodes are not always easy to access (e.g., the parenchyma of the parotid gland), thus increasing risk of serious injury.
Patients with positive biopsy results should undergo complete lymph node dissection or receive adjuvant radiation therapy of the nodal basin. Dissection is the treatment of choice, but radiation has also been used successfully in patients at high risk of complications of surgery61 (Tables 2 and 3). Radiation has been shown to be as effective as surgery in controlling subclinical disease.62
Excision of palpable nodes combined with irradiation of the basin has been recommended when multiple or massive node involvement or extracapsular spread might be present,63 although some authors have found that radiotherapy alone controls disease as well as surgery.64
If it is decided not to perform SLNB in MCC, the nodal basin should be irradiated as a precautionary measure (Tables 2 and 3, Fig. 1).
When nodal irradiation is chosen (elective, adjuvant, or radical) all stations in the region of drainage should be included.65,66
Disseminated DiseaseThe treatment of metastatic MCC relies mainly on chemotherapy, but surgery or radiation therapy may also be used.67
MCC responds to a great range of chemotherapies. Drugs used to treat small cell lung cancer are the ones most often used in MCC.68 The regimens that give the best results combine cisplatin, or carboplatin, with etoposide or use topotecan in monotherapy. The combination of cyclophosphamide (or epirubicin) and vincristine is also given but is more toxic. The level of evidence supporting these therapies is low (2A), but as MCC is highly chemosensitive, treatment usually leads to a satisfactory partial or complete response. The effect, however, is short-term: in most cases and the disease progresses, recurring within 4 to 15 months.69 It must be emphasized that there is no clear evidence that chemotherapy prolongs survival.70,71 Furthermore, a mortality rate of 7.7% has been attributed to chemotherapy-related toxicity.72 Therefore, the decision to provide adjuvant or palliative treatment of this type, particularly in older or immunocompromised patients, should be grounded in the clinical judgement of a multidisciplinary team.
Surprisingly good outcomes have been reported in isolated cases of MCC treated with c-kit inhibitors in recent years.73 Although CD117 expression was detected by immunohistochemistry in 95% of MCC tumors in one series, mutations on the c-kit receptor could not be demonstrated.74 While the receptor is expressed in MCC, therefore, it is not activated. Disease progressed in most patients in a series of 23 cases when 400mg/d of imatinib was given; a partial response was observed in only 1 patient.21 In another trial, disease only became stable in a single patient given imitinib.75 The use of imatinib is therefore not recommended at present.
Some interesting treatment approaches that take into the account the relationship between MCC and MCPyV are under study in vitro or in vivo. An approach that includes antiviral agents might prove useful in treating this tumor in the future.
Furthermore, MCC, like melanoma, might logically respond to immune therapy for several reasons: the incidence is higher, and prognosis worse, in immunocompromised patients; MCC is known to regress spontaneously; an intense CD8 inflammatory infiltrate is associated with a better prognosis, as is the lack of a primary skin tumor; and MCPyV has been found in the genome of tumor cells and its presence is associated with a better prognosis. It is interesting, therefore, that various pharmacologic pathways are now being explored in patients with metastasis. Therapies under study are the anti-PD-L1 drug pembrolizumab, anti-PD-1, anti-CTLA-4 (ipilimumab), and the interleukin 12 gene plus a plasmid DNA vaccine. Clinical trials with these new candidate approaches will clarify whether any offer new benefits.
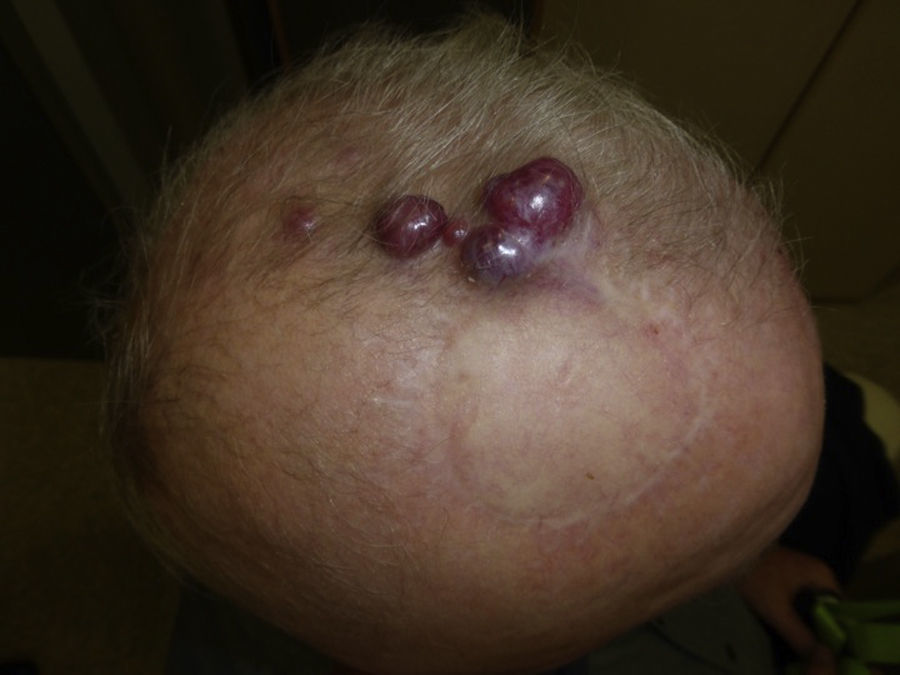
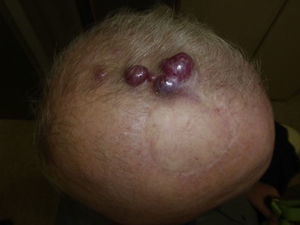
Follow-upMCC recurrence (Fig. 4) presents within 3 years of diagnosis in 90% of cases. Metastasis usually affects the lymph nodes (27%–60% of cases) or skin (9%–30%); less often the lung (10%–23%), liver (13%), bone (10%), brain (6%), bone marrow (2%), or other organs (6%) may also become involved.14,72
The NCCN recommendations call for follow-up visits every 3 to 6 months in the first 2 years after diagnosis and later every 6 to 12 months. Follow-up visits should include a physical examination and thorough palpation of lymph nodes; tests ordered will depend on the clinical findings.1 PET-CT is the imaging technique of choice for detecting metastasis. If it is unavailable, however, magnetic resonance imaging or conventional CT may be useful. Analysis of alkaline phosphatases, as markers of liver disease, and serology to detect MCPyV are also recommended, and are scheduled at the same time as examinations. Zageretal.8 suggested that, as in melanoma, ultrasound studies of the regional lymph nodes should be done every 3 to 6 months in patients at high risk of locoregional recurrence. After the first 2 years of follow-up, these studies may be done on a 6-to-12 month schedule.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Dr. Rebeca Alcalá and Dr. David Llorca, who made this review possible.
Please cite this article as: Llombart B. Actualización en el carcinoma de células de Merkel: claves de las técnicas de imagen, factores pronóstico, tratamiento y seguimiento. Actas Dermosifiliogr. 2017;108:98–107.