The development and commercialization of glucose sensors and insulin pumps has revolutionized the management of diabetes. These devices have been linked to multiple cases of contact dermatitis in recent years, however, giving rise to a growing interest in identifying the sensitizing allergens. Isobornyl acrylate was clearly identified as one of the main allergens responsible for contact dermatitis among users of the FreeStyle glucose sensor and was subsequently removed from the product ingredients. Remarkably, however, it is still used in most other sensors on the market. The common adhesive ingredients colophony and abietic acid derivatives have also been shown to be sensitizing agents. New components under study, such as dipropylene glycol diacrylate, N,N-dimethylacrylamide, and triethylene glycol methacrylate have recently been identified as allergens, though they are not commercially available for clinical testing. The benefits offered by glucose sensors and insulin pumps may be offset by sensitization to product ingredients, in some cases forcing discontinuation and diminishing quality of life. Dermatologists should play a role in this clinical and research scenario, offering case-by-case guidance to endocrinologists on skin care and possible alternatives for patients with glucose sensors and insulin pumps who develop contact dermatitis. They should also collaborate with the manufacturers developing these devices.
El desarrollo y comercialización de los sensores de glucosa y las bombas de insulina han supuesto una revolución en el control de los pacientes diabéticos. En los últimos años se han detectado múltiples casos de dermatitis de contacto relacionados con estos dispositivos médicos, con el creciente interés sobre los alérgenos responsables de la sensibilización. Isobornil acrilato fue sin duda el alérgeno principal del dispositivo FreeStyle, motivando al fabricante a modificar la composición eliminando este alérgeno. Curiosamente, este alérgeno está presente en casi todos los sensores comercializados. La colofonia y derivados del ácido abiético desempeñan un papel relevante en cuanto al adhesivo. Recientemente aparecen nuevos componentes identificados como alérgenos, no comercializadas, como el dipropilene glicol diacrilato, la N,N-dimetilacrilamida, o el metacrilato de trietilenglicol, que están siendo foco de estudio. El impacto positivo que tiene el uso de estos dispositivos puede verse mermado por la sensibilización a uno de sus ingredientes, obligando en ocasiones a abandonar el dispositivo, y por ende, restando calidad de vida. El dermatólogo debe posicionarse respecto al estudio dirigido de estos pacientes, dando soporte a los servicios de endocrinología, con la finalidad de orientar tanto el cuidado de la piel como las alternativas posibles, especialmente con la colaboración de los fabricantes.
Diabetes mellitus (DM) is a chronic disease in which sustained hyperglycemia has a negative impact on multiple organs, mainly the nervous and vascular systems. In 2019, data from the World Health Organization reported 1.5 million deaths were due to DM, with nearly half of them occurring before the age of 70. After the arrival of insulin and oral hypoglycemic agents, glucose sensors and insulin pumps emerged, which were a game-changer in the management of type 1 diabetic patients. Although they are subject to the regulations of the European Parliament and Council 2017/745 (EU) on the manufacture of medical devices, in recent years, numerous cases of allergic contact dermatitis (ACD) to different allergens have been reported. This justifies that dermatologists should be knowledgeable and updated whether they are specialized in the management of contact dermatitis or not.
The incidence of DM is still on the rise in Europe, with 5% and 8.8% increase estimates for type 1 and type 2 DM, respectively, in 2015. Specifically in Spain, the prevalence of diabetes has reached 14.8%.1 Early diagnosis and regular patient follow-up are essential to prevent complications such as hypoglycemia and diabetic ketoacidosis. Currently, we have medical devices available that have been designed to facilitate the monitoring and management of diabetes, and prevent complications associated with this disease,1,2 including continuous glucose monitoring systems, or glucose sensors, and subcutaneous continuous insulin infusion systems, or insulin pumps.1,2 Currently, in Spain, only a few models have been funded by the National Health System in some autonomous communities and certain risk groups.1
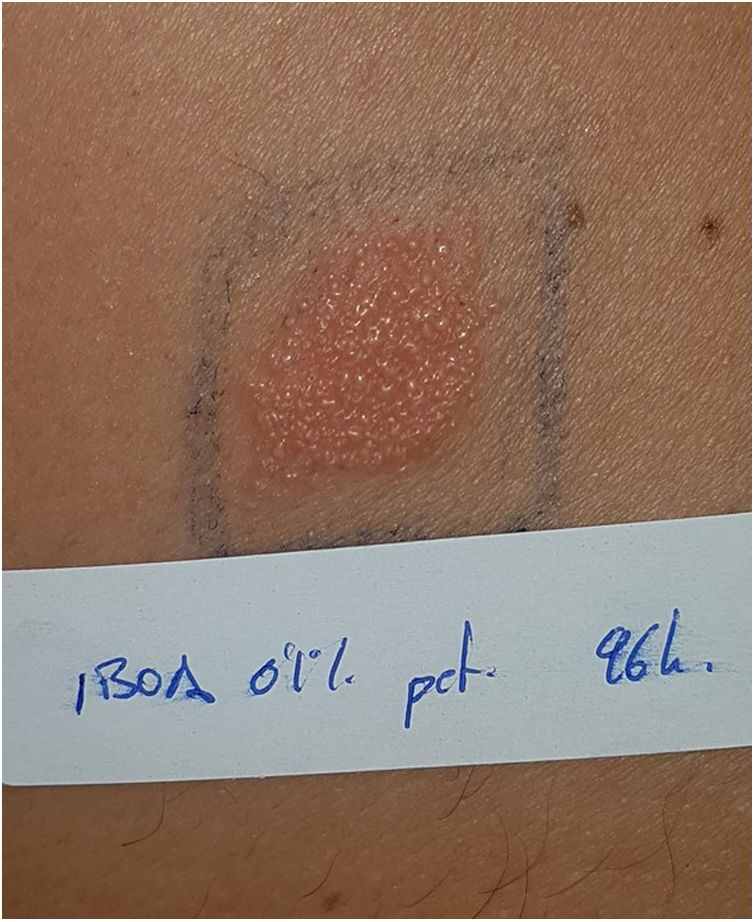
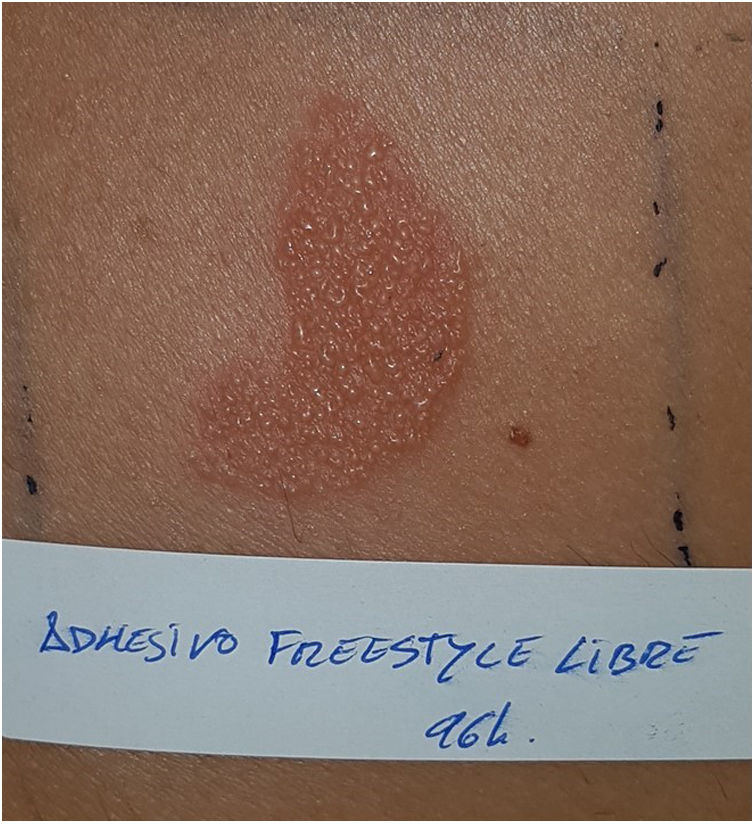
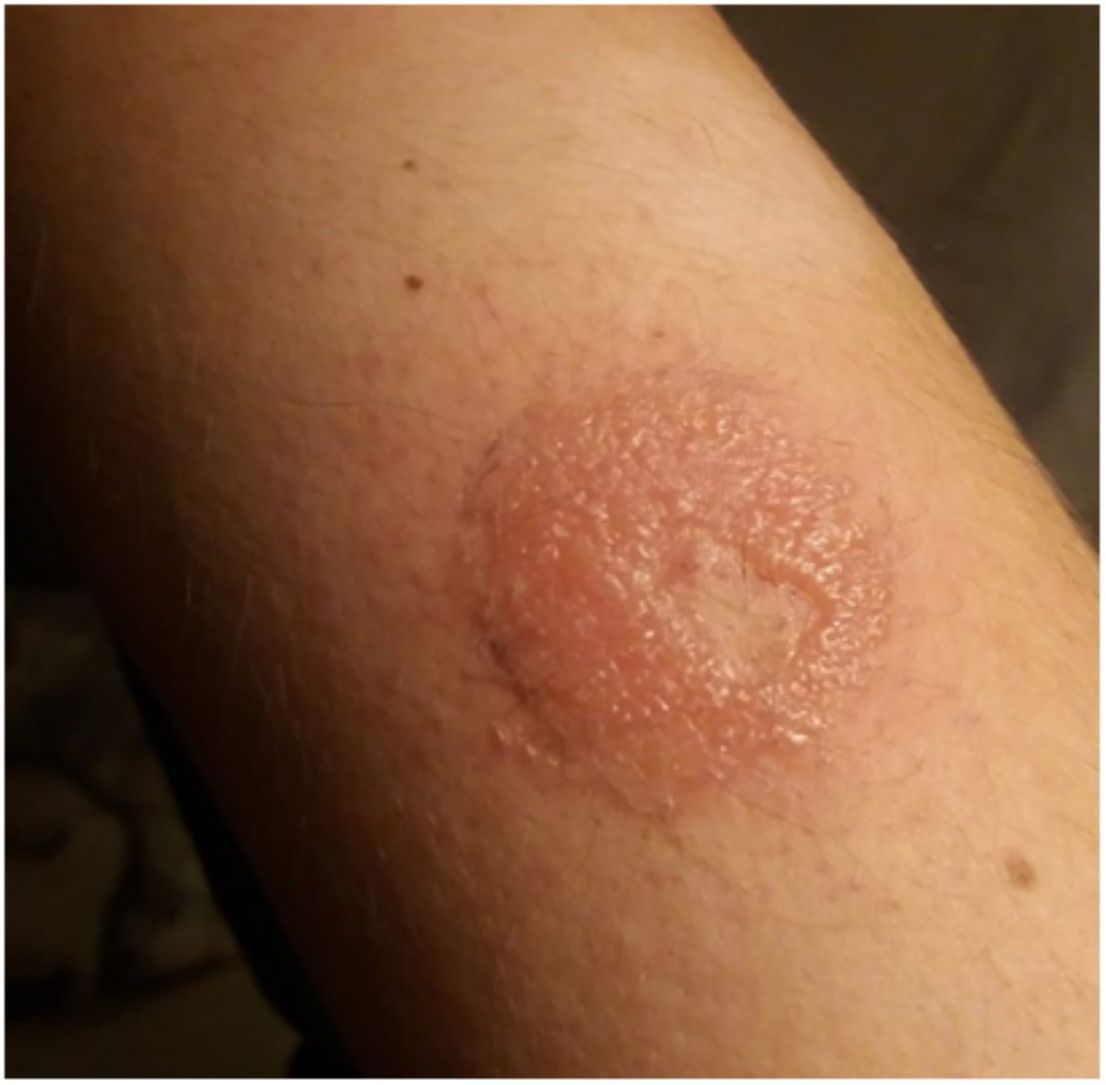
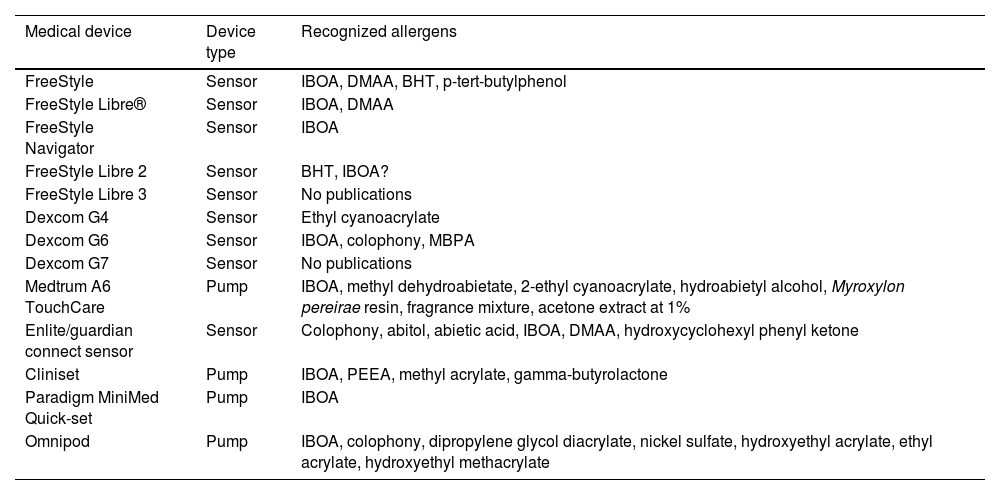
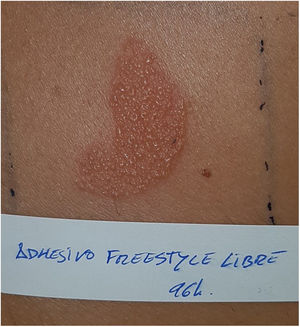
Continuous glucose monitoring systemsFreeStyle Libre®FreeStyle Libre® (Abbott Laboratories) was the first glucose sensor that became available in the market. This medical device, which is applied to the skin with an adhesive for up to 14 days, continuously measures glucose levels.1,3 Shortly after its market launch, cutaneous reactions associated with this device consistent with ACD started being reported. Subsequent studies confirmed isobornyl acrylate (IBOA; CAS registry no. 5888-33-5) as the most relevant allergen causing FreeStyle Libre®-induced ACD.3,4 Some studies estimate that 5.5% of the patients wearing FreeStyle Libre® experience ACD, being 3.8% of the cases attributed to IBOA.5 For diagnostic purposes, a patch test with the adhesive and IBOA in petrolatum at a concentration of 0.1% is needed being cross-reactivity to other acrylates limited or null.1,6,7 The main therapeutic option is to avoid the allergen, that is, switch to a different continuous glucose monitoring system. Some authors suggest replacing the blood glucose sensor with Eversense® (Ascensia), or Dexcom® (Medtronic) to eliminate cutaneous reactions.2,6 However, the use of these devices does not guarantee eliminating the risk of developing contact dermatitis since they also contain potentially sensitizing allergens.7 Other variants of the FreeStyle®, such as the FreeStyle Navigator® I and II, are also available and contain IBOA among their components (Figs. 1 y 2).
Subsequent analysis using gas chromatography-mass spectrometry (GC-MS) indicated the presence of N,N-dimethylacrylamide (DMAA; CAS registry no. 2680-03-7) in the FreeStyle Libre® sensor, considered the second allergen associated with coming into contact with this device.8,9
It is likely that the IBOA and DMAA found in the FreeStyle Libre® sensor come from the adhesive used to join the top and bottom parts of the sensor plastic cover. The high degree of concomitant reactions to DMAA and IBOA is probably due to simultaneous exposure to these substances when using the FreeStyle Libre®.8 Because of the structural differences reported, cross-reactivity between IBOA and DMAA seems unlikely.8 According to a recent multicenter trial, the frequency of sensitization to IBOA and DMAA in Spain is lower than that reported in other European series.10 Also, we cannot discard that other previously unknown sensitizers could also play a key role here. Since both IBOA and DMAA are components of adhesives used for medical devices, such as glucose sensors and insulin pumps, patch tests should be performed whenever ACD is suspected in association with medical devices.10
The cases reported to date describe patients allergic to the Freestyle Libre® device who also tested positively to a sesquiterpene lactone mix, which contains equimolar concentrations of alantolactone (0.033%; CAS registry no. 546-43-0), costunolide (0.033%; CAS registry no. 553-21-9), and dehydrocostus lactone (0.033%; CAS registry no. 477-43-0).9,11,12 The presence of all 3 components in the glucose sensor could not be demonstrated through GC-MS.11 Although their chemical functional groups are very similar, cross-reactivity between sesquiterpene lactone and IBOA seems unlikely due to their different spatial structures.1,11 Therefore, cosensitization, rather than cross-reactivity, remains the most likely explanation since IBOA and sesquiterpene lactone have a common precursor, camphene.9
FreeStyle Libre 2®In 2019, a change in the manufacturing process of the Freestyle Libre® eliminated IBOA from the sensor plastic housing. This second generation is called Freestyle Libre 2®.13 Despite observations that patients with known ACD to IBOA could tolerate the Freestyle Libre 2®, cases of ACD continued to be reported.13 Afterwards, a new allergen—2,6-di-tert-butyl-4-cresol (CAS registry no. 128-37-0)—was detected as part of the new adhesive of the Freestyle Libre 2®, which was not present in the original Freestyle Libre®.13 This chemical substance acts as an antioxidant in food, petroleum-derived products, rubber, plastics, and cosmetics. Allergic reactions to 2,6-di-tert-butyl-4-cresol have been reported after the use of drugs and cosmetics. Although the widespread use of 2,6-di-tert-butyl-4-cresol was considered a safe antioxidant at the concentrations typically used, we should mention that the devices remain on the skin of diabetic patients for days or even weeks, leading to more intense contact than usual and an increased likelihood of contact allergy.1,2,14 In 2022, the FDA approved a new version of the FreeStyle Libre® (the FreeStyle Libre 3®). To date, no cases of contact dermatitis have ever been reported, and its composition is still unknown.
Dexcom G4 / G5 MobileCases of ACD associated with the Dexcom G4® Platinium / G5 Mobile glucose sensor have been reported too. Cyanoacrylate-based adhesives are some of the components associated with these continuous glucose monitoring systems.15 In 2016, the first case of a child with Dexcom G4®-induced ACD was reported.16 Patch tests revealed a positive reaction to hydroxy-cyanoacrylate. Subsequent studies reported two cases of ACD due to 2-ethyl cyanoacrylate (CAS registry no: 7085-85-0), considered the primary allergen causing excom G4® Platinium/G5 Mobile-induced ACD.16 2-ethyl cyanoacrylate is used as the adhesive for the sensor and is widely used in household and industrial adhesives. It can also be found in cosmetics for nail application, as well as in adhesives for artificial nails and dental restoration products. Patients primarily become sensitized through prolonged exposure. However, exposure to acrylates contained in gel nails, nail polishes, and adhesives can also trigger these skin reactions.17 On the contrary, it has been reported that the housing of Dexcom G4® Platinium / G5 Mobile and its adhesive do not contain IBOA and are well-tolerated by patients allergic to the Freestyle Libre® due to this allergen.15
Dexcom G6®Since 2020, severe cases of ACD associated with the Dexcom G6® glucose sensor have been reported, which is the new Dexcom version that does not include 2-ethyl cyanoacrylate in its composition.18 However, new allergens causing ACD have been reported, including 2,20-methylenebis (6-tert-butyl-4-methylphenol) monacrylate (CAS registry no. 128-37-0), IBOA, and colophony derivatives. 18–20
2,20-methylenebis (6-tert-butyl-4-methylphenol) monacrylate is a thermal stabilizer and antioxidant used in a wide range of adhesive and plastic materials.18 Unlike traditional stabilizers and antioxidants, this substance is an effective remover of alkyl radicals. This property is especially useful in high-temperature processes and environments with low oxygen levels, such as during the initial mixing of adhesives. A patch test with a 1.5% concentration is advised to achieve diagnosis.18
In 2022, the latest version called Dexcom G7® was introduced. It is a disposable single-use device, which is smaller in size than the previous 6th, 5th, and 4th-generation systems (G6, G5, and G4).21 The G7 system also differs from these previous systems in that the transmitter and sensor are supplied as an integrated unit.20 Due to its recent release, no cases of ACD have ever been reported associated with its use, and it is unknown whether any changes have ever been made to its components.
Enlite/Guargian connect sensorThe Enlite® glucose sensor is a medical device developed as a continuous glucose monitoring system for diabetic patients. It consists of a catheter associated with an adhesive film that adheres to the skin.22 The Guardian Connect (Medtronic)® is reusable, rechargeable, and connects to the Enlite® glucose sensor sending data to the reader, often a mobile phone. Passanisi et al. described the occurrence of ACD associated with the Enlite® glucose sensor due to the presence of rosin derivatives as confirmed by the manufacturer.23 Like the FreeStyle Libre® glucose sensor and the OmniPod® insulin pump, IBOA is also present in the Enlite® glucose sensor.23 Former studies confirm the presence of IBOA in extracts from Enlite® sensors, but not in adhesive patch extracts. However, small amounts of IBOA can also be present in these patch extracts.23 Additionally, Enlite® sensor extracts suggested the presence of DMAA, similar to the FreeStyle Libre®, and hydroxycyclohexylphenylketone.
MicroneedlesOne of the most important components of continuous glucose monitoring devices are microneedles (MN). Their composition should be taken into consideration in patients with ACD. Metal is the most common material used in MN because of how easy it is manufacture.24 Stainless steel and titanium are some of the metals used for their strength. Noble metals such as gold and silver are often applied to MN for sensor performance enhancement.24,25 Silicon is another frequently used material known for its excellent mechanical resistance. However, the fabrication cost is a key factor limiting its use.24 In recent years, polymer MN have become popular because they are biocompatible, biodegradable, mechanically resistant to cutaneous insertion, and easy to manufacture.24 A wide variety of polymers, such as polymethyl methacrylate, polylactic-co-glycolic acid, polyglycolic acid, cyclic olefin copolymer, polyvinylpyrrolidone, polyvinyl alcohol, methacrylate hyaluronic acid, and SU-8 have been described in the manufacturing process of MN.24
Insulin pumpsInsulin pumps are systems for the continuous and controlled administration of insulin.1,2. Typically, they consist of a reservoir connected to a cannula or needle attached to the skin with adhesives. The cannula or needle is often changed every 2 to 3 days to prevent complications mainly due to insulin flow blockage, but also others like ACD,1,2 wich due to the common replacement and possibility of alternating its location, often has less clinical relevance.1,26. Therefore, the actual prevalence of ACD may be underestimated, and sometimes can be taken for irritant dermatitis due to the plastic components, which stresses the importance of conducting patch tests in the presence of clinical suspicion.27
AutoSyringe®Designed in 1976, the AutoSyringe® had a plastic tube which connected to the needle. Epoxy resins were often used as an adhesive to bring these components together, leading to cases of ACD occurring as early as one day after implantation as described in reports from the 1980s, some associated with concomitant sensitizations to p-tert-butylphenol formaldehyde resin (CAS registry no. 98-54-4), and fragrances.28
Cliniset, Clini Softs, Set per microinfusione®Shortly after the arrival of the AutoSyringe, cases of ACD started being reported with other insulin pumps built by Pharmaplast in Denmark. In particular, significant positive reactions were found vs IBOA and multiple acrylates, even at 0.001% dilutions, in the case of phenoxy(polyethyleneoxy)ethylacrylate (CAS registry no. 92-50-2).29–31 Again, these cases were mostly associated with the glues and adhesives used, but also to the plastic catheter in the case of methyl acrylate and gamma-butyrolactone.32 The presence of these compounds was verified through GC-MS.33
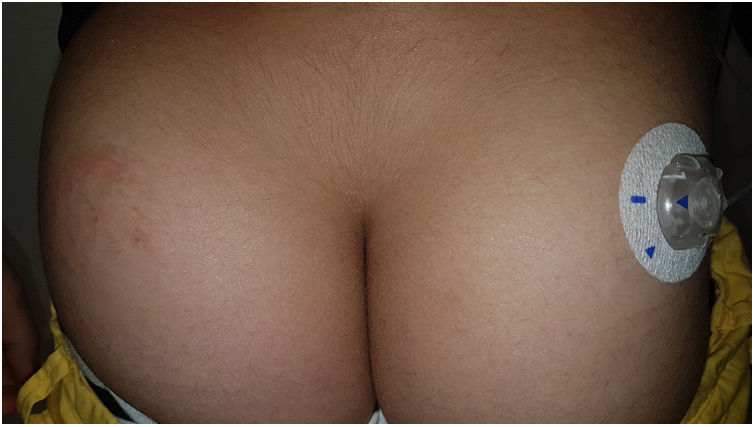
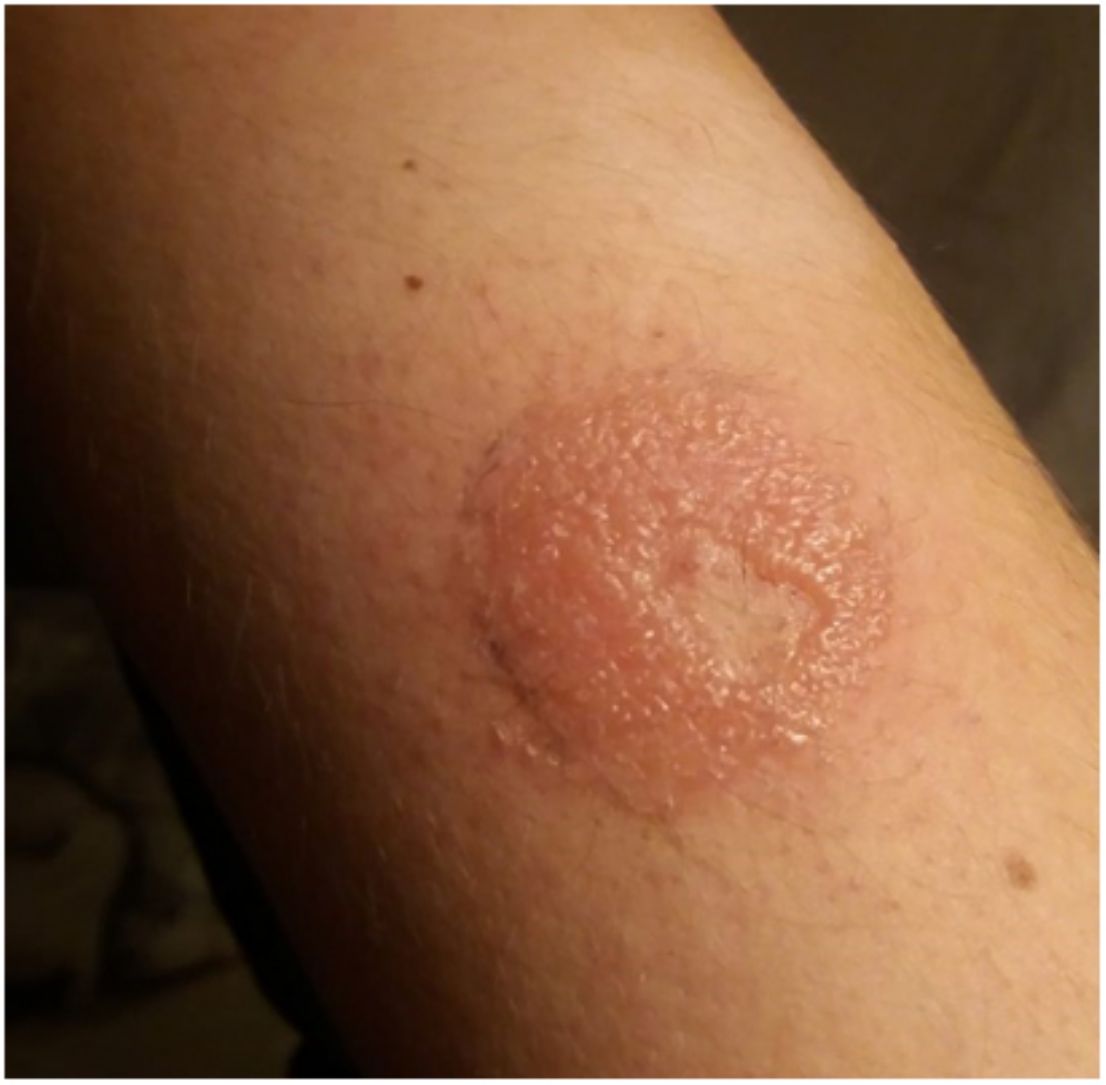
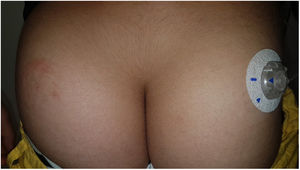
OmniPod (Ypsomed)®The first patch insulin pump (2010) to enter the European market was the OmniPod (Ypsomed)®. The first allergen associated with its development was IBOA, which was actually more associated with the process of bringing different parts together, a mechanism triggered by heat, rather than its adhesion to the skin.34 Additionally, one woman tested positive for nickel, potassium dichromate, and rosin,23 and another, who had previously used permanent nail polish, tested positive for other allergens, including three other acrylates (hydroxyethyl acrylate, ethyl acrylate, hydroxyethyl methacrylate), which are often present in nail polishes.35,36 In a recent observational trial, rosin has once again been reported as one of the main allergens that should be taken into consideration in these devices (Fig. 3), especially when used together with the Enlite® device.37
Paradigm MiniMed Quick-set®In recent years, new insulin pumps such as the Paradigm MiniMed Quick-set® have arrived, and several cases of ACD have been reported associated with its use. The first two patients had used the FreeStyle Libre® sensor and tested significantly positive for IBOA—detected in the device—a fact that was confirmed by other authors.38.
Medtrum A6 TouchCare®This is a recently marketed reservoir pump that attaches directly to the skin, without a catheter connecting to the needle. Back in 2020, the first case of allergic dermatitis was reported in a type 1 diabetic woman who showed reactivity to the adhesive patch and, in particular, to IBOA 0.3%, hydroabietyl alcohol, Myroxylon pereirae resin, a mixture of fragrances, and acetone extract 1%. GC-MS confirmed the presence of other compounds, including derivatives of rosin (also confirmed by the manufacturer).39
Allergens not included in sensors or pumpsWhen studying patients with continuous glucose monitoring devices, or insulin pumps, it is essential to consider not only the components of the devices but also the potential causes for ACD that are frequently used by these patients, such as chlorhexidine, betadine, topical antibiotics, metals, preservatives, fragrances, or nail acrylics. However, it is challenging to determine the possible association between these allergens and exposure to glucose sensors, or insulin pumps, because patients often do not have a clear relationship, either current or past, with these identified contact allergies.
Additionally, it is common for patients to use adhesive wipes to prevent sensors from falling off. Among the components of some of these adhesive wipes, such as the Skin Tac Wipe®, potential allergens like isopropanol and rosin can be found. The composition of Conveen Prep® includes isopropyl alcohol, butyl ester of PVM/MA copolymer, and isobutyl acetate of saccharose.
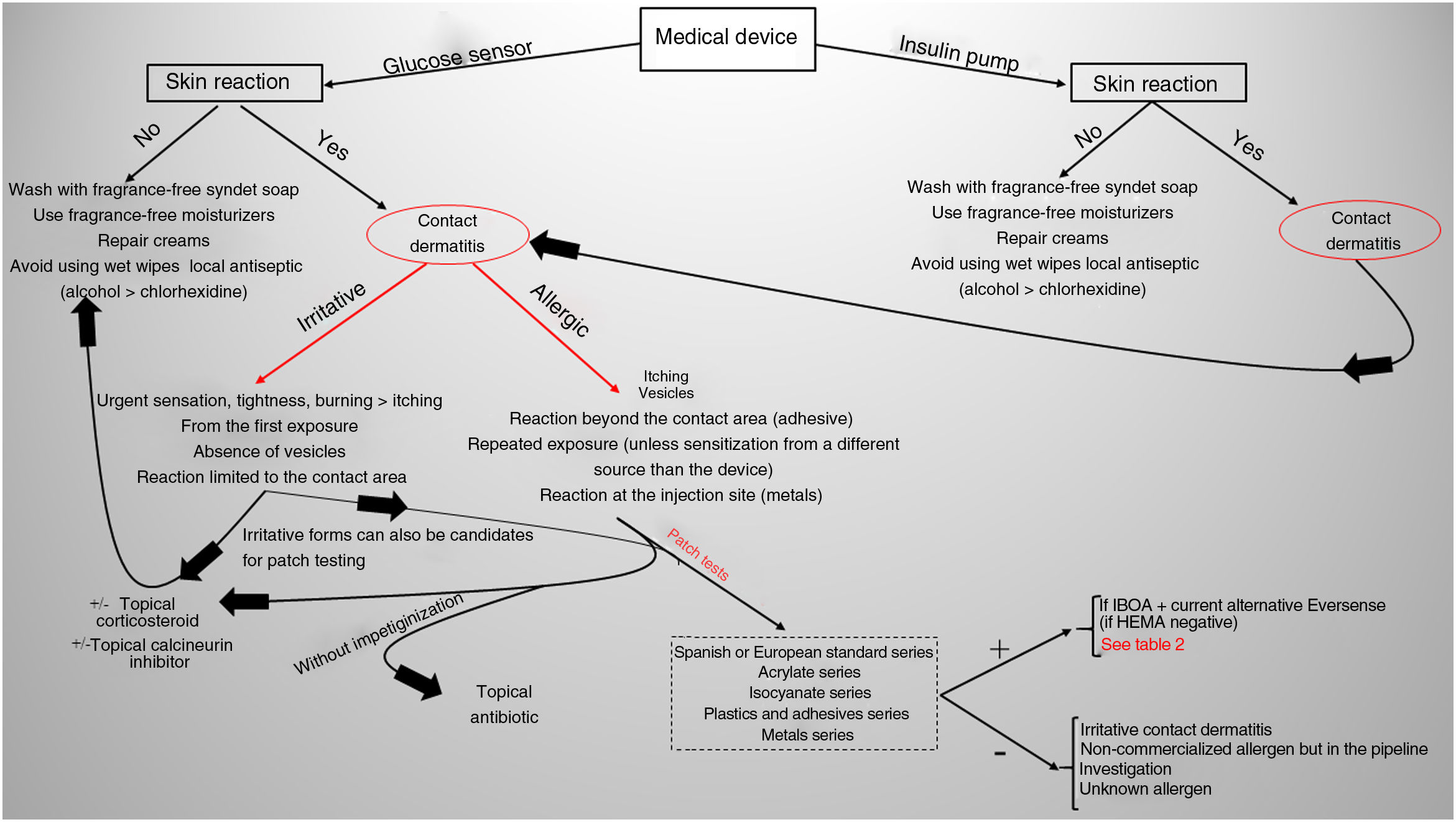
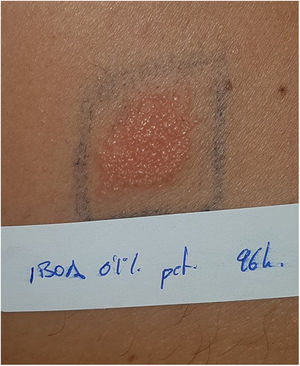
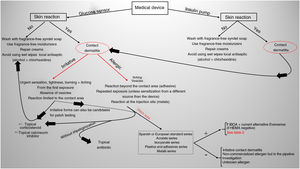
Current situation and reflectionsThe use of glucose sensors and insulin pumps will grow exponentially with their new indication for type 2 DM, which represents 95% of diabetic patients. This will undoubtedly have a positive impact on the quality of life of these patients, and reduce complications associated with the disease through better glycemic control. However, it will also increase the incidence of new cases of ACD. Currently, we still don’t know the actual prevalence of ACD associated with these medical devices in our country, or the allergens involved. Collaboration and transparency from manufacturers on the composition of each sensor are crucial to facilitate patch testing. We may be at a turning point where the implementation of specific panels for studying reactions to glucose sensors could be valuable (including the allergens shown in Table 1). Additionally, standardizing the method with the same allergens would help determine their true impact on our patients. However, this is almost impossible to date because most allergens involved so far are not commercially available (Table 2 includes those published to date). The decision to withdraw or replace the device in each patient should be made on an individual basis, although there may be potential limitations due to the current lack of knowledge on the exact composition of each device. Recently, the presence of IBOA in a glucose sensor that had not reported containing this allergen has been published. It is evident that we find ourselves caught in an important and delicate moment where dermatologists—whether dedicated to contact dermatitis or not—will have to actively participate in decision-making on what to do in each case (Fig. 4).
Allergens recommended by the authors that should be included in the panel of medical devices (glucose sensors and insulin pumps) for diabetic patients with contact dermatitis.
| Standard series (includes colophony, sesquiterpene lactone mix) |
| Metal series |
| Acrylate series (including isobornyl acrylate) |
| Hydroabietyl alcohol |
| Methyl dehydroabietate (not commercially available) |
| Isocyanates (specific panel) |
| Benzoyl peroxide |
| N,N-dimethylacrylamide (not commercially available) |
| Hydroquinone and derivatives (if leukoderma) |
| 2-ethyl cyanoacrylate |
| 2,6-di-tert-butyl-4-cresol (BHT) |
| Hydroxyethyl methacrylate (not commercially available) |
| Triethylene glycol methacrylate (not commercially available) |
| N,N-dimethylacrylamide (not commercially available) |
Devices available and known allergens/published in the current scientic medical literatura available.
| Medical device | Device type | Recognized allergens |
|---|---|---|
| FreeStyle | Sensor | IBOA, DMAA, BHT, p-tert-butylphenol |
| FreeStyle Libre® | Sensor | IBOA, DMAA |
| FreeStyle Navigator | Sensor | IBOA |
| FreeStyle Libre 2 | Sensor | BHT, IBOA? |
| FreeStyle Libre 3 | Sensor | No publications |
| Dexcom G4 | Sensor | Ethyl cyanoacrylate |
| Dexcom G6 | Sensor | IBOA, colophony, MBPA |
| Dexcom G7 | Sensor | No publications |
| Medtrum A6 TouchCare | Pump | IBOA, methyl dehydroabietate, 2-ethyl cyanoacrylate, hydroabietyl alcohol, Myroxylon pereirae resin, fragrance mixture, acetone extract at 1% |
| Enlite/guardian connect sensor | Sensor | Colophony, abitol, abietic acid, IBOA, DMAA, hydroxycyclohexyl phenyl ketone |
| Cliniset | Pump | IBOA, PEEA, methyl acrylate, gamma-butyrolactone |
| Paradigm MiniMed Quick-set | Pump | IBOA |
| Omnipod | Pump | IBOA, colophony, dipropylene glycol diacrylate, nickel sulfate, hydroxyethyl acrylate, ethyl acrylate, hydroxyethyl methacrylate |
BHT, 2,6-Di-tert-butyl-4-cresol; DMAA, N, N-dimethylacrylamide; IBOA, isobornyl acrylate; MBPA, 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate; PEEA, phenoxyethyl(ethylenoxy)ethyl acrylate.
Contact dermatitis due to medical devices for diabetic patients has gained special interest in recent years. The new indication will increase its use, and we suspect a growing incidence that will require us to become familiar with this topic to offer the study and subsequent recommendations in the most accurate and up-to-date way possible.
Conflicts of interestNone declared