Psoriasis is a recurrent chronic inflammatory disease mediated by the immune system, with the most common phenotype (> 80% of cases) being plaque psoriasis.1–3 The main goal of psoriasis treatment is to maintain clear skin for as long as possible and prevent the onset of potential associated comorbidities.4–6
Classical systemic drugs—including dimethyl fumarate—are first-line therapies for moderate-to-severe psoriasis.7 The safety and efficacy profile of dimethyl fumarate has been demonstrated in various studies and in the routine clinical practice,8–10 with its main limitation being the proper management of titration and its adverse effects (AEs), which are mostly mild and manageable.
Patient Support Programs (PSPs) can provide valuable support to the dermatologist's clinical work, offering patients information and training for optimal disease management and treatment adherence.
PSP Skilarence® Responde is a program that offers patients who start dimethyl fumarate therapy comprehensive services provided by the nursing staff. This service includes support for drug titration, dietary-nutritional advice, recommendations for managing AEs, and answers to treatment- and disease-related questions, always coordinated with the hospital dermatology unit. PSP Skilarence® has a few limitations, such as the absence of a control group or the potential bias of positive responses when asking about program satisfaction via phone calls.
This PSP starts after a patient voluntarily and proactively requests enrollment. Patients formalize their inclusion by signing an informed consent form. This PSP includes regular follow-up calls during the drug titration phase and provides patients with the option for proactive contact as needed while enrolled in the PSP.
The aim of this study is to present the characteristics of patients enrolled in the PSP through descriptive analyses of data collected during the study period.
For analysis purposes, the usual treatment regimen included in the technical data sheet was used as a reference, and maintenance was defined as the date from which—after, at least, 9 weeks on therapy—the dose of dimethyl fumarate remained constant for, at least, 6 consecutive weeks.
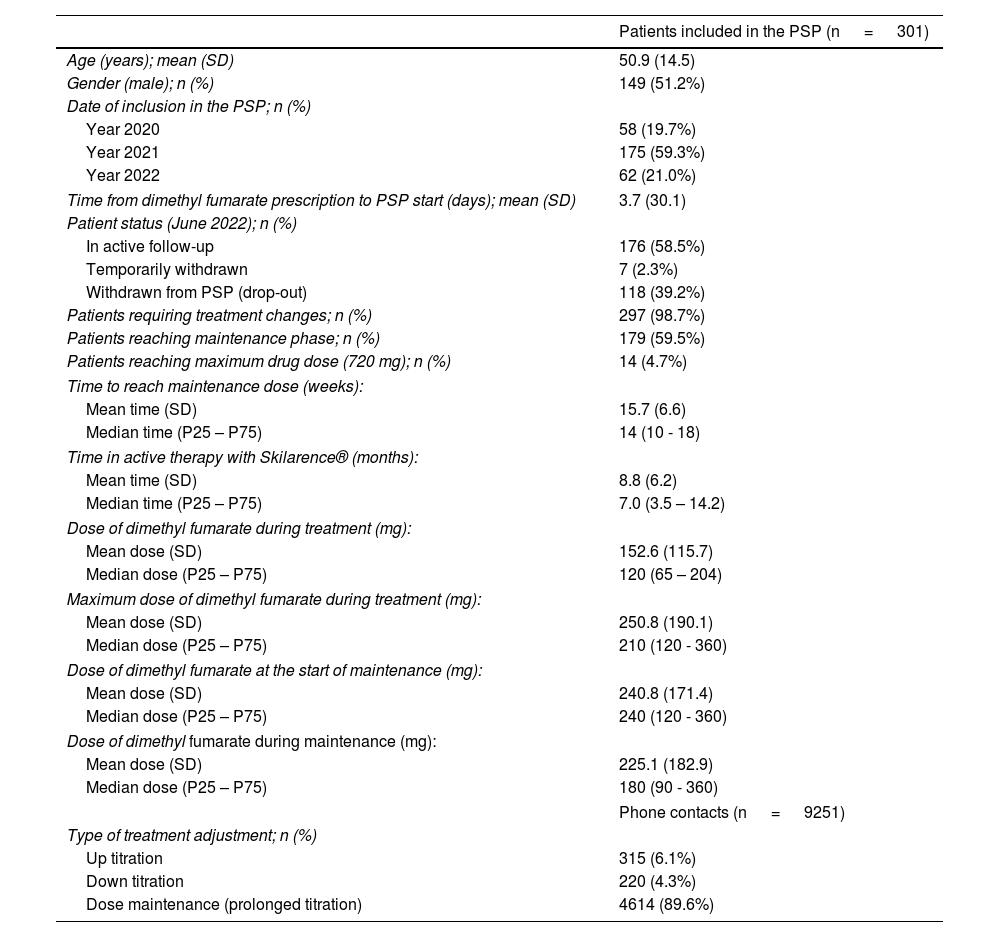
From June 2020 through June 2022, the PSP included a total 301 patients, with a mean age (standard deviation [SD]) of 50.9 (14.5) years (51.2% were men (Table 1).
Characteristics and treatment management of patients included in the Skilarence® Responde PSP.
| Patients included in the PSP (n = 301) | |
|---|---|
| Age (years); mean (SD) | 50.9 (14.5) |
| Gender (male); n (%) | 149 (51.2%) |
| Date of inclusion in the PSP; n (%) | |
| Year 2020 | 58 (19.7%) |
| Year 2021 | 175 (59.3%) |
| Year 2022 | 62 (21.0%) |
| Time from dimethyl fumarate prescription to PSP start (days); mean (SD) | 3.7 (30.1) |
| Patient status (June 2022); n (%) | |
| In active follow-up | 176 (58.5%) |
| Temporarily withdrawn | 7 (2.3%) |
| Withdrawn from PSP (drop-out) | 118 (39.2%) |
| Patients requiring treatment changes; n (%) | 297 (98.7%) |
| Patients reaching maintenance phase; n (%) | 179 (59.5%) |
| Patients reaching maximum drug dose (720 mg); n (%) | 14 (4.7%) |
| Time to reach maintenance dose (weeks): | |
| Mean time (SD) | 15.7 (6.6) |
| Median time (P25 – P75) | 14 (10 - 18) |
| Time in active therapy with Skilarence® (months): | |
| Mean time (SD) | 8.8 (6.2) |
| Median time (P25 – P75) | 7.0 (3.5 – 14.2) |
| Dose of dimethyl fumarate during treatment (mg): | |
| Mean dose (SD) | 152.6 (115.7) |
| Median dose (P25 – P75) | 120 (65 – 204) |
| Maximum dose of dimethyl fumarate during treatment (mg): | |
| Mean dose (SD) | 250.8 (190.1) |
| Median dose (P25 – P75) | 210 (120 - 360) |
| Dose of dimethyl fumarate at the start of maintenance (mg): | |
| Mean dose (SD) | 240.8 (171.4) |
| Median dose (P25 – P75) | 240 (120 - 360) |
| Dose of dimethyl fumarate during maintenance (mg): | |
| Mean dose (SD) | 225.1 (182.9) |
| Median dose (P25 – P75) | 180 (90 - 360) |
| Phone contacts (n = 9251) | |
| Type of treatment adjustment; n (%) | |
| Up titration | 315 (6.1%) |
| Down titration | 220 (4.3%) |
| Dose maintenance (prolonged titration) | 4614 (89.6%) |
PSP: Patient Support Program; SD: standard deviation.
* Variables collected may contain missing data, not considered in the analysis.
Patients enrolled in the PSP Skilarence® Responde remained in the program for a mean (SD) of 10.3 (6.6) months, with a mean (SD) treatment adherence of 8.8 (6.2) months. Most patients (88.5%) completed their first 9 weeks of treatment (standard titration), while 63.7% remained in the PSP for, at least, 6 months.
A total of 9251 calls were recorded in the PSP, most of which were scheduled (6244 calls; 67.5%) and associated with the administration of the dimethyl fumarate regimen (n=5238; 56.6%). The mean (SD) number of scheduled calls per participant was 20.8 (12.6).
Since the start of treatment, 98.7% of patients required some type of adjustment (with respect to the recommendations made in the dimethyl fumarate technical data sheet), and in 89.6% of them, this involved dose maintenance or extension of the drug titration phase (Table 1).
Overall, a total of 179 patients (59.5%) completed the drug titration phase in a mean (SD) time of 15.7 (6.6) weeks (median of 14 weeks). The mean dose (SD) at the time of reaching maintenance was 240.8 (171.4) mg (Table 1 and Fig. 1).
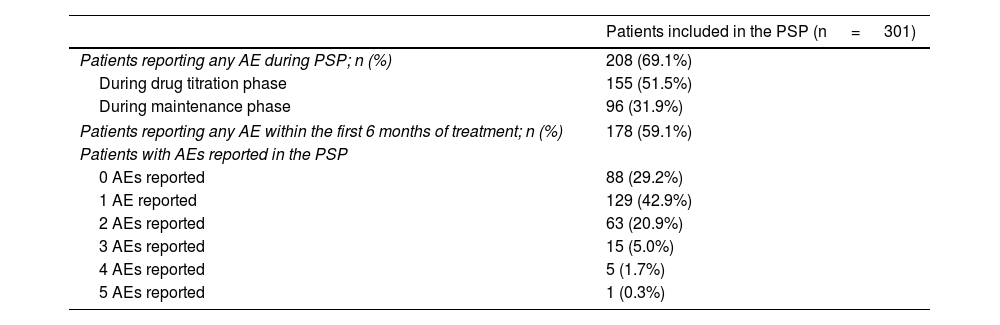
A total of 51.5% of patients experienced AEs during drug titration, decreasing to 31.9% during maintenance. Gastric discomfort was the most frequently reported AEs, primarily during titration, with a total of 125 patients being affected (41.5%), followed by redness, with 65 patients being affected (21.6%). During maintenance, lymphopenia was the most frequently reported AE, affecting a total of 38 patients (12.6%) (Table 2).
Adverse effects reported by patients on dimethyl fumarate included in the Skilarence® Responde PSP.
| Patients included in the PSP (n = 301) | |
|---|---|
| Patients reporting any AE during PSP; n (%) | 208 (69.1%) |
| During drug titration phase | 155 (51.5%) |
| During maintenance phase | 96 (31.9%) |
| Patients reporting any AE within the first 6 months of treatment; n (%) | 178 (59.1%) |
| Patients with AEs reported in the PSP | |
| 0 AEs reported | 88 (29.2%) |
| 1 AE reported | 129 (42.9%) |
| 2 AEs reported | 63 (20.9%) |
| 3 AEs reported | 15 (5.0%) |
| 4 AEs reported | 5 (1.7%) |
| 5 AEs reported | 1 (0.3%) |
| AEs reported in the PSP (n = 325)* | |||
|---|---|---|---|
| Type of AE reported, n (%) | Total | Titration | Maintenance |
| Gastric discomfort (general) | 151 (50.2) | 125 (41.5) | 26 (8.6) |
| Redness | 65 (21.6) | 65 (21.6) | 9 (3.0) |
| Lymphopenia | 49 (16.3) | 11 (3.7) | 38 (12.6) |
AE: adverse events; PSP: Patient Support Program.
The mean (SD) patient satisfaction with dimethyl fumarate treatment was 7.2 (2.6) points, while satisfaction with the service provided by the PSP Skilarence® Responde was rated with a mean (SD) score of 9.8 (0.5) points out of a total of 10.
Regarding satisfaction as perceived by dermatologists (n=17), the mean (SD) satisfaction with treatment was 8.2 (1.3) points, while satisfaction with the PSP was 9.7 (0.5) points, out of a total of 10.
The data collected in the PSP Skilarence® Responde suggest that for dimethyl fumarate therapy, proper management of initial dose titration is critical, which may require around 14 weeks, and establishing an individualized maintenance dose is necessary in each case, rarely requiring the maximum drug dose (720mg/day). Nursing support in patient management is key to assisting patients in adapting to the drug, and the role of the dermatologist is crucial in defining a progressive process to achieve mid- to long-term goals.
FundingThe program is entirely funded by Almirall and has dedicated staff from IQVIA Information.
Conflicts of interestM.I. Navarro works for IQVIA. T. Guilà works for Almirall. G. Osorio and M.L. Alonso-Pacheco declared no conflicts of interest whatsoever.
The authors wish to thank the health care professionals who contributed to the inclusion of patients in the PSP Skilarence® Responde, and the involvement of the nursing staff who support and advise patients with moderate-to-severe psoriasis from the health centers. The authors also appreciate the collaboration of David Jiménez, Albert Rafels Ybern, and David Asensio Torres.